A decade after the discovery of BRCA1 and BRCA2 genes, enormous research advances have been made. A high breast, ovarian cancer risk has been established pressing for a prevention decision. But as surgical and nonsurgical options abound, increases parallel the uncertainty about a right choice.
On one hand, prophylactic surgery -bilateral mastectomy (BM), bilateral salpingo-oophorectomy (BSO) and resection of both breasts and ovaries, dramatically reduces cancer risk at the organ(s) targeted by the BRCA mutated genes but at the cost of all disadvantages of a surgical approach. On the other, surveillance strategies providing an excellent quality of life (QoL) represent women's preferences. Lifelong preservation -due incomplete penetrance or modifying the genetic risk (tamoxifen)- or surgical resection only when early-stage cancer becomes clinically evident, is an ideal goal. But is research sufficient for integration into clinical practice without risks?
Wide variation in risk estimates, diverse impacts of surgical and nonsurgical preventive measures on survival and QoL, as well as lacking of randomized controlled trial, make a right decision too complicated and extremely challenging. As new data have become available, what is the preventive intervention that provides the best risk-benefit ratio in clinical practice regarding risk reduction, survival and quality of life?
When the BRCA11 and BRCA2 genes were discovered a decade ago, researchers were overoptimism for a great success against breast cancer. They thought that the identification of these high-penetrance genes would lead to understanding not only the inherited BRCA pathway but also that of most common sporadic (nongenetic) breast cancer. Understanding carcinogenesis of both inherited and sporadic breast cancer would lead to effective strategies for prevention of the most common female malignancy with major impacts on women's public health.3 We know now that these expectations have not been fulfilled. But important advances have been made in identifying women with high genetic risk of breast cancer. As genetic testing is widely available and the number of women who carry BRCA1 or BRCA2 mutations steadily increases and prevention options abound, increases also the uncertainty about choosing an ideal prevention strategy. Currently, the key question of women with these mutations and their physicians is to choose from multiple surgical and nonsurgical interventions available, the best that provides a high cancer protection index with the less adverse effects on quality of life (QoL). What is this optimal prevention?
Inherited BRCA-driven tumors account for approximately 5% to 10% of all new breast cancers. This is a small proportion, but considering that this year 275,000 women will receive a new diagnosis of breast cancer in the United States[4] and approximately 130,000 women in Europe, we can estimate that with appropriate prevention strategy about 20,250 to 40,500 BRCA carriers annually could avoid the risk of breast cancer. This incidence reduction gain for public health with effective prevention may become greater in the future.
Indeed, with population aging given and other risk factors -women's lifestyle and use of hormone replacement therapy (HRT)- which it is unlikely to change in the next decades, incidence of sporadic and inherited breast cancer will continue to increase. Therefore, strategies for improving prevention of inherited breast cancer maximizing survival benefits and minimizing QoL adverse effects will attract a major research and clinical interest in the next years.
It has been well established that women with inherited mutations in the BRCA1 or BRCA2 (BRCA) genes face an evident high lifetime risk of developing breast and ovarian cancer. To prevent this risk several surgical and nonsurgical approaches have been developed. Ideally, prevention strategy should provide the best combination of cancer protection, survival and quality of life (QoL). Goal of prophylactic surgery is to eliminate risk of cancer, by removing the organ(s) targeted by the BRCA mutated genes, namely breasts and ovaries, before the disease can clinically be detected. By contrast, preservation or surgical resection of these target organs only when screening-detected early-stage cancer occurs represents the principal aim of surveillance strategies.[5]
Theoretically, both surgical and nonsurgical approaches appear to be rational. On one hand surgery can nearly completely eliminate risk of cancer by complete removal of target organ(s) but at the cost of all disadvantages of a surgical procedure. On the other, many carriers of inherited mutations may strongly benefit from surveillance strategies for three reasons. First, because of incomplete penetrance of BRCA genes, a proportion of BRCA carriers will never develop cancer without any intervention. Second, another fraction of women can benefit by modifying this genetic risk. Such potentially non-genetic modifiers include tamoxifen and reproductive or other factors. Third, simultaneously, screening all BRCA carrier it could be prevented late cancer diagnosis, which is associated with high mortality risk. Both surgical and nonsurgical approaches are creative thoughts for prevention. For making a good decision however, in clinical practice essential is the availability of data.
But information is scanty. Randomized controlled trials (RCTs), that elegantly fulfill the strict criteria of rules of evidence-based medicine, for apparent reasons will never become available even in the future. Thus, prevention management should be guided by other nonrandomized reports.[6] Furthermore, the evident high risk of cancer developing for BRCA carriers urgently suggests the need for interventions. Therefore, a prevention decision should be made on the basis of available information.
Wide variation in risk estimates, diverse impacts of surgical and nonsurgical preventive measures on survival and QoL and insufficient data, make prevention decision too complicated and challenging. As new data have become available over the last year regarding lifetime risk,[7] the efficacy and limitations of surgical and nonsurgical procedures,[8-10] and decision analysis[11] for carriers of BRCA mutations, critical analysis on the light of these new data together with previous information may help women and their physician to deal with the dilemma of preventive choosing.
Ten years after the discovery of BRCA genes and the establishment of hereditary breast and ovarian cancer syndrome (HBOCS), an explosion of research and scientific interest on understanding the molecular mechanisms of BRCA pathway has been noted. However, the great expectations, that this elucidation could lead to the development of effective chemoprevention even of the most common noninherited (sporadic) cancer and thereby to major potential impact on women's public health has been proven elusive. However, surprising insights into the BRCA genes' biological function abound.[12-14] Although the BRCA genes themselves appear unconnected to common, nonhereditary cancers, emerging evidence suggests that defects in other parts of the BRCA pathway might be critical not only driving breast cancer but other cancers as well.[13,14]
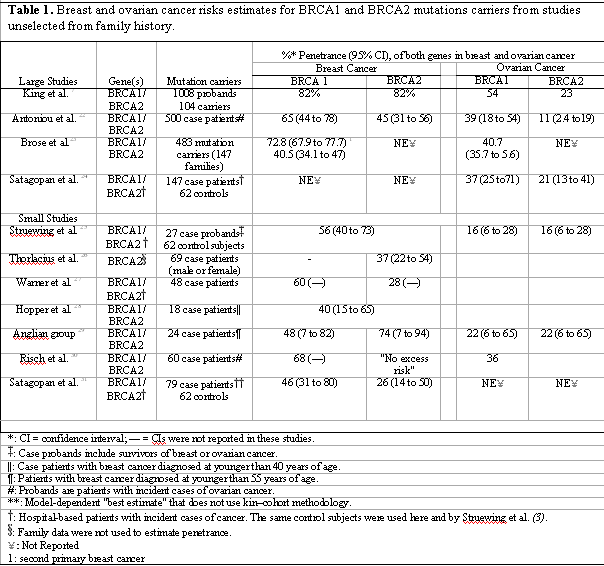
Wide variation. Most, but not all, women with inherited mutations that affect one allele of either BRCA1 or BRCA2, will develop breast and/or ovarian cancer. This risk varies considerable between 40% to 85% for breast cancer and 11% to 65% for ovarian cancer.[7,12,15,16] This variation is depended on mutated gene (BRCA1 or BRCA2 and different mutations in the same gene), family history (strong or weak), as well genetic and external factors which modify genetic risk.[12] Early studies, which used high-risk families, showed very high breast and ovarian cancer risk for BRCA mutation carriers.[17-21] Subsequent studies, to offset such biases, used population-based incident cases of cancer. (Tables 122-32). Risk estimates for BRCA carriers are higher than in general population, but lower[15,16] to those reported from multiple-cases families.[17-21] Considering all this information, it was believed that among women with the deleterious BRCA mutations only those with a strong family history have a really high risk of cancer whereas those without such a family history are likely to possess a much lower risk for breast cancer.[33]
New study - higher risk
However, a recent study published in Science[7] may overturn this widely accepted opinion. In the New York Breast Cancer Study (NYBCS), 1,008 New York-area Ashkenazi Jewish women diagnosed with incident, breast cancer between 1996 and 2000 were enrolled. Of these female probands 104rriers from low-incidence families (10.3%) carried an ancient mutation in BRCA1 or BRCA2. Exactly half (50%) of these 104 patients with breast cancer and inherited mutations were from low-incidence families with no breast or ovarian cancer among mothers, sisters, grandmothers, or aunts. In nearly all these low-incidence families, BRCA1 and BRCA2 mutations proved to be inherited from fathers. In these low-incidence families, breast cancer was also evaluated for second-, third, and fourth-degree relatives (grandmothers, aunts, and cousins) with BRCA1 or BRCA2 mutations. By comparing age, family history, cancer status, and other factors, King et al.[7] determined that the overall lifetime risks for breast cancer were 82% for BRCA1 or BRCA2 carriers and for ovarian cancer were 54% for BRCA1 carriers and 23% for BRCA2 mutation carriers. The most important finding, from the clinical point of view however, as opposed to the available data, is the very high risk of breast and ovarian cancer among mutations carriers regardless of family history. In other words, the evidence for the presence of BRCA1 or BRCA2 mutations in a woman represents a very high cancer risk whereas family history has a rather little, nonsignificant, impact on a carrier's cancer risk.
 |
The findings of this study provide two potentially important clinical implications. First, validating the very high lifetime risk of breast and ovarian cancer for women with BRCA mutations and at least one relative with breast cancer, as conclude Levy-Lahad and Plon in their accompanying perspective article[34] provide convincing evidence for the urgent need of effective preventive intervention. Second, and exciting new, is that all women with BRCA mutations, even those who are distant relatives from both mother's side and father's side, of a breast cancer case face a high lifetime risk. If this finding be confirmed by other studies will strongly affect and probably change genetic test counseling and prevention decision for surgical or nonsurgical approach.
|
SURGICAL AND NONSURGICAL APPROACHES |
Prophylactic surgery includes bilateral mastectomy (BM), bilateral salpingo-oophorectomy (BSO) and resection of both breasts and ovaries. By removing the BRCA-targeted organ(s), surgery can dramatically reduce an overall cancer risk.[35] Indeed, although BRCA mutated genes are found in every cell in the body of carriers, they target nearly exclusively breast tissue, and to a lesser extent, the ovary, and fallopian tube. Small appears to be the risk of other cancers, such as pancreatic, colorectal and stomach of women with inherited mutations.[23,36,37] Thus, complete surgical removal of breasts and/or ovaries may result in a very high protection from all BRCA-driven cancers. However, surgery does not fulfill women's preferences and expectations on organs preservation. Thus, considering incomplete penetrance, potential risk reduction by and screeing availability for early detection surveillance strategies with or without chemoprevention have been developed.
Prophylactic surgery
Early on, prophylactic BM in women with family history of breast cancer had been suggested. With the availability of genetic testing, surgery is performed only to women with evident BRCA mutations. In a recent report,[8] 483 women with BRCA1/2 mutations were studied. After a mean follow-up of 6.4 years breast cancer was diagnosed in two (1.9%) of 105 women who had bilateral prophylactic mastectomy and in 184 (48.7%) of 378 matched controls who did not have the procedure. Rebbeck et al. conclude that bilateral prophylactic mastectomy reduces the risk of breast cancer in women with BRCA1/2 mutations by approximately 90%.[8] In a prospective study of 139 women with BRCA1 or BRCA2 mutations, after a mean follow-up of 3 years, breast cancer was developed in 8 of 63 women who had elected surveillance but in none of the 76 BRCA mutations carriers who had undergone prophylactic surgery.[38] High morbidity in up to 30% after BM and reconstruction[39] and adverse effects on psychosocial status have been reported. However, recent studies show low rate of complications after reconstruction, less than 10%.[40] Furthermore, the majority of women were satisfied with their decision, more those women with reconstruction, to undergo BM and were not experiencing abnormal levels of psychological distress, low levels of sexual activity, or difficulties with body image.[41]
Surveillance Screening
Research efforts are underway to develop nonsurgical interventions for high-risk women for an improved QoL without increasing cancer mortality. However, data on surveillance-based strategy are few and suggest major limitations. The addition of magnetic resonance imaging (MRI), to the modest efficacy regular clinical breast examination and annual mammography, can increase the rate of early detection, but a substantial proportion of BRCA carriers still experiencing late diagnosis. In a latest prospective study,[9] at a mean follow-up of 2.9 years, [23] of 358 women with BRCA mutations developed ductal or invasive breast cancer. MRI significantly increased the rate of early detection, but the rate of tumors larger than 1 cm was 43% and only 63% of patients had node-negative disease.9 More frequent mammographic and MRI screening, for example every six months, might decrease late diagnosis with early detection of "interval cancers" increasing thereby early-stage cancer. But at present there is not such evidence. Because of its low specificity, MRI increases the unneeded additional investigations and biopsies.[9]
Non-genetic modifiers.
Tamoxifen, an antiestrogenic modifier of genetic risk,[12] can reduce breast cancer risk. But tamoxifen is effective only in estrogen-receptors (ER) positive tumors in the general population and probably also in BRCA-associated tumors. Since most BRCA1-driven tumors are ER-negative, antiestrogens have no impact in contrast to BRCA2-driven tumors the majority of which is ER-positive. Indeed, in the NSABP-P1 trial, tamoxifen chemoprevention reduced breast cancer incidence in BRCA2 carriers but not to those with BRCA1 mutations.[42] Whether BRCA1-driven tumors truly not respond to hormonal manipulation as prevention therapy -a controversial topic- as well as whether other selective estrogen receptor modulators (SERM) and aromatase inhibitors are more effective than tamoxifen in BRCA1 or BRCA2 carriers is evaluated in various ongoing chemoprevention trials. Much more research is needed to identify the small proportion among BRCA1 and BRCA2 carriers, which is sensitive or not respectively for a guided to chemoprevention. Other non-genetic factors may also modify genetic risk.[12] Pregnancy, physical exercise as adolescent, healthy weight[7] and breast-feeding[43] are all factors that may delay the onset or even reduce the risk of cancer.
Data on breast cancer prevention indicate that BM can dramatically reduce breast cancer risk by at least 90%.[8] This rate can further be increased by special consideration to complete surgical removal of breast tissue. Despite intensive research, surveillance at present cannot be integrated into clinical practice without a substantial risk of delayed diagnosis at advanced-stage breast cancer and mortality.
|
OVARIAN CANCER PREVENTION |
Ovarian cancer is less common than breast cancer, but it is among the most lethal cancers.[44] Although among BRCA carriers the risk of ovarian cancer (11-23%) is lower than that of breast cancer (Table 1), it is much higher than that of 1-2% in the general population.[44] Since breast tumorigenesis is causatively linked with estrogen,[3] it is not surprising that bilateral salpingo-oophorectomy (BSO) reduces risk of both ovarian cancer and breast cancer. This protective effect of BSO has been suggested by previous small studies and decision analysis[45,46] and it is confirmed by recent reports. In a large, retrospective analysis of 551 BRCA carriers, BSO reduced the risk of ovarian cancer by 96% and of breast cancer by 53% at a mean follow-up of 9 years[47] Similar findings provided a prospective study of 170 BRCA carriers. During a mean follow-up of 2 years, incidence of ovarian or peritoneal cancer and breast cancer was significantly greater in women who selected surveillance than in those who choose to undergone BSO.[48] Laparoscopically performed BSO is associated with low morbidity (4%)48 and all benefits of a minimal-access surgery. High efficacy, minimal invasiveness and ineffective surveillance available all explain an increasing trend towards BSO in recent years. Intensive screening with ultrasonography and CA-125 in BRCA carriers for an ovarian cancer detection at early stage has been failed.[48] A recent analysis of studies published between 1998 and 2003 provided a convincing biological basis to explain the failure of such a screening. Most ovarian cancers in women with inherited mutations are high-grade serous cancers, and these are infrequently screen at an early stage.[10] On the basis of this screening ineffectiveness, the authors conclude that BSO appears to be the most effective approach to reduce ovarian-cancer risk and mortality in women with high-genetic risk.[10]
Hormone replacement therapy (HRT) after BSO. Concerns and caution suggests the paradox that on one hand with BSO we remove the vast majority of endogenous hormone effects to prevent ovarian and breast cancer and on the other we prescribe HRT. Hormonal manipulation to manage frequently powerful menopausal symptoms, may increase breast cancer risk. Early BSO, as soon as possible after completion of childbearing, is currently recommended on the basis of data available.[10,11,45-48] But what are the consequences of such a HRT? Particularly after the publication of the Women's Health Initiative data demonstrated an increased risk of breast cancer, coronary disease and thromboembolic events in general population,[49] the uncertainty about similar adverse effects on BRCA carriers has been increased. This dilemma to use or not HRT after BSO in BRCA carriers was evaluated in a recent Markov decision analysis.[11] There was no significant increase of adverse effects among those carriers who received HRT and those who did not receive. Armstrong et al.[11] conclusion is that BSO and HRT can be recommended in BRCA carriers after completion of childbearing. Despite these findings, in an accompanying editorial, Garber and Hartman from Dana Farber Cancer Institute in Boston, suggest caution in the widespread of HRT.[50] Their proposal for better surveillance-based strategies to replace prophylactic surgeries and adverse effects on QoL is undoubtedly a reliable future research goal. But at the moment, pressing is the need for advice about the most effective cancer protection in current clinical practice.
|
CHOOSING PREVENTION INTERVENTION |
Limitations
Treatment or prevention decisions should be meet the criteria and rules of evidence-based science. In the case of BRCA carriers with an absence of evidence from RCTs, it is easy to say that no medical decision can be made. But the evident very high genetic risk of BRCA carriers, urgently press for as soon as prevention decision. Therefore, prevention choice will be based on data from nonrandomized reports.6 Prospective studies are few and with short-term follow-up.[38,48] Observational reports have all the limitations of retrospective evaluation and follow-up is less than 10 years indicating that long-term effects of surgical and nonsurgical interventions are unknown. All these limitations should be considered and reveal how complicated is an appropriate prevention choice Penetrance and impacts of prevention strategies on cancer risk, survival, and QoL are the key criteria considered for making a good decision.[5] Table 2 summarizes data regarding these variables.[7,22-24] A risk-benefit ratio considering all these parameters is essential for decision-making. It is however, elusive to believe that a certain prevention option can fully meet all these expectations together in each individual woman. On one hand, nonsurgical procedures provide good QoL but may be associated with increased risk of advanced-stage cancer and mortality, on the other surgery ensures a very high protection from cancer but it is associated with all the disadvantages of invasiveness, non-reversibility, surgical morbidity and adverse effects on QoL.[51]
Research promise on surveillance strategies
Ideally, surveillance may result in lifelong preservation of target organs or its surgical resection only when cancer becomes clinical evident. This approach clearly reflects women's preference. Unfortunately, individual women who benefit from close screening and/or chemoprevention cannot be identified. But even with the most modern imaging technology available, accurate prediction of individual women with incomplete penetrance, response to chemoprevention or detection at a very early-stage of carcinogenesis cannot be approached. This identification can emerge only due advances in biological and molecular research. Understanding signalling pathways and identifying genetic and environmental modifiers appears to be the most rational way to determine BRCA carriers who will benefit from surveillance.[3] Genomics- based diagnostic techniques, such as gene-array analysis and research focused on evaluating molecular pathways[52] will help to identify those BRCA carriers who will develop breast or ovarian cancer. Exciting findings using gene-expression profiles based on tissue-array analysis in clinically evident breast cancer are already available.[53,54]
Rational of surgery
Despite the reliable focus of research on developing effective nonsurgical interventions for improving QoL, in current clinical practice prophylactic surgery is clearly superior to surveillance. Surgery reduces the risk of cancer by at least 90%.[8,47] The high protective index despite a possible over estimation,[55] decision analyses,[11,45] and advances in surgical techniques including laparoscopic approaches, which have led to low morbidity and less adverse effects on QoL ,[40,41] have resulted in an increasing acceptance of surgical prevention among both oncologists and women with BRCA mutations. Indeed, recent data suggest high levels of satisfaction of women for their decision to choose a surgical intervention that was associated with low impact on their psychosocial status.[41]
Which surgical procedure is the best? Of three surgical approaches available, radical removal of both breast and ovaries provides the highest cancer protection. But because of its major invasiveness and complications is preferred by few women. Most BRCA carriers have to decide between BM and BSO, but it is a great challenge since these two procedures have various effects on efficacy and QoL.[56] Both BRCA1 and BRCA2 carriers have a higher risk of breast cancer. But the efficacy of BSO to reduce risk of both breast by 50% and ovarian cancer by 90%,[45-48] the more extensive surgery and increased morbidity with BM and reconstruction, and the advantages of BSO with laparoscopic approach, along with the absence of effective ovarian-cancer screening, suggest the superiority of BSO.[57] Disadvantage of BSO is the need for HRT to treat serious symptoms after this early surgical menopause with unknown long-term effects. Although no significantly increased risk of breast cancer, heart disease and thromboembolism by the use of HRT in BRCA after BSO has been obtained in a recent decision analysis,[11] considering HRT-associated adverse effects in general population,[49] a careful and individualized decision about HRT prescribing is advised.[50]
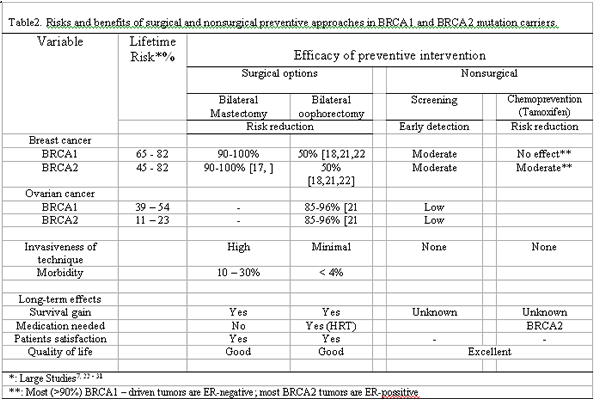 |
Separate decision for BRCA1 and BRCA2 carriers appears useful. For BRCA1 carriers there is little or no room for discussion. The evident very high lifetime risk, consistently suggested by Large studies,[7,22-24] of both breast cancer (65-82%) and ovarian cancer (39-54%) (Tables 1, 2) along with failure response to tamoxifen chemoprevention[42] suggest the urgent need for surgical prophylaxis. Compared with BRCA1 carriers, women with BRCA2 mutation face a lower probability of developing cancer in the breast (45-82%) or ovary (11-23%). But this risk remains high, much higher than in general population, and despite the tamoxifen effectiveness -most BRCA2 are ER positive tumors- the uncertainty about cancer mortality persists.
Expanding indications for BRCA genetic test
The power of genetic testing for stratifying individuals into low-risk and high-risk groups, determinant fordecision making; the availability of effective preventions and a wide availability of genetic test in manly medical centers have expanded the applications of BRCA assessment. Since 5% to 10% of women with a new breast cancer diagnosis carry BRCA mutations and initial surgical treatment of breast cancer differs between carriers and non-carriers, genetic risk assessment may affect surgical decision.[58] Neither breast-conserving surgery, which is the current standard treatment for early-stage breast cancer[59] nor mastectomy, are appropriate procedures for BRCA carriers. The mutated genes continue to target the available breast tissue, after these procedures namely the preserved breast and/or the contralateral breast. This hypothesis is confirmed by a most recent study in which women diagnosed with unilateral breast cancer, had a 40% risk of developing contralateral breast cancer within 10 years.[60] Genetic counselling and BRCA test, especially for young women and those with family history with a new breast cancer diagnosis is strongly advised before treatment, because it may change decision for initial of surgery.
With the discovery of BRCA genes enormous progress has been made and multiple prevention options have been developed. However, right individualized decision at the right time is elusive. Intensive research on nonsurgical prevention strategies provides the best promises for the future. But at present, demands and preferences of women with BRCA mutations to avoid removal of breasts and/or ovaries cannot be satisfied without risk. Surveillance strategy is associated with a high risk of late diagnosis of breast cancer and particularly of ovarian cancer and mortality in some women, who despite advances in molecular research and image technology cannot be identified.
In current clinical practice, rational is a surgical approach. Prophylactic surgery does not cure the cause, namely the inherited mutations. But with complete resection of target organ(s), surgery provides a very high efficacy regarding cancer prevention and survival. As surgery evolves, postoperative morbidity rates and adverse effects on QoL have drastically been reduced. Laparoscopic bilateral salpingo-oophorectomy after completion of childbearing providing today the best risk-benefit ratio, is increasingly acceptable by both doctors and their patients toward improving primarily survival and QoL.
1. Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266:66-71.[ISI][Medline]
2. Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995;378:789-792. [Erratum, Nature 1996;379:749.][CrossRef][ISI][Medline]
3. Agnantis NJ, Paraskevaidis E, Roukos DH. Carcinogenesis of breast bancer: Advances and applications. Gastric Breast Cancer 2004;3: 5-14. (Accessed October 12, 2004, at http://www.gastricbreastcancer.com/breastcancer_abs.asp
4. Jemal A, Tiwari RC, Murray T. Cancer Statistics, 2004. CA Cancer J Clin 2004; 54:8-29.
5. Agnantis NJ, Paraskevaidis E, Roukos DH. Preventing breast, ovarian cancer in BRCA carriers: Rational of prophylactic surgery and promises of surveillance. Ann Surg Oncol (in press). [Editorial].
6. Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process, 2004. (Accessed July 9, 2004, at http://www.ahrq.gov/clinic/ajpmsuppl/harris3.htm.)
7. King MC, Marks JH, Mandell JB; New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003 Oct 24;302(5645):643-6.
8. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004 Mar 15;22(6):1055-62.
9. Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351:427-437.
10. Hogg R, Friedlander M. Biology of epithelial ovarian cancer: implications for screening women at high genetic risk. J Clin Oncol. 2004 Apr 1;22(7):1315-27.
11. Armstrong K, Sanford Schwartz J, Randall T, et al: Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: A decision analysis. J Clin Oncol 2004; 22:1045-1054.
12. Narod SA. Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer. 2002 Feb;2(2):113-23. Review.
13. Couzin J. The twists and turns in BRCA's path. Science 2003 Oct 24;302(5645):591-3.
14. Venkitaraman A. Tracing the network connecting BRCA and Fanconi anaemia proteins. Nat Rev Cancer 2004 April; 4: 266-276.
15. Nathanson KN, Wooster R, Weber BL. Breast cancer genetics: What we know and what we need. Nat Med 2001; 7: 552-556.
16. Georgiou, I, Fatouros M, Arampatzis I, Batsis H, Briasoulis E, Paraskevaidis E, Agnantis NJ, Roukos DH. Evaluating cancer risks in BRCA mutation carriers. Gastric Breast Cancer 2003; 2(2): 45-51.
17. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 1998;62:676-89.
18. Ford D, Easton DF, Bishop DT, Narod SA, Godgar DE. Risks of cancer in BRCA1 mutation carriers. Lancet 1994;343:692-5.
19. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 1995;56:265-71.
20. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 1998;62:676-89.[Medline][CANCERLIT]
21. Narod SA, Ford D, Devilee P, Barkadottir RB, Lynch
HT, Smith SA, et al. An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Am J Hum Genet 1995;56:254-64.
22. Antoniou A, Pharoah PD, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003 May;72(5):1117-30.
23. Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 2002;94:1365-72.
24. Satagopan JM, Boyd J, et al. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res. 2002 Dec;8(12):3776-81.
25. Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997;336:1401-8.
26. Thorlacius S, Struewing JP, Hartge P, Olafsdottir GH, Sigvaldason H, Tryggvadottir L, et al. Population-based study of risk of breast cancer in carriers of BRCA2 mutation. Lancet 1998;352:1337-9.
27. Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 1999;91:1241-7.
28. Hopper JL, Southey MC, Dite GS, Jolley DJ, Giles GG, McCredie MR, et al. Population-based estimate of the average age-specific cumulative risk of breast cancer for a defined set of protein-truncating mutations in BRCA1 and BRCA2. Australian Breast Cancer Family Study. Cancer Epidemiol Biomarkers Prev 1999;8:741-7.
29. Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer 2000;83:1301-8.
30. Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 2001;68:700-10.
31. Satagopan JM, Offit K, Foulkes W, Robson ME, Wacholder S, Eng GM, et al. The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev 2001;10:467-73.
32. Levy-Lahad E, Catane R, Eisenberg S et al. Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel: frequency and differential penetrance in ovarian cancer and in breast-ovarian cancer families. Am J Hum Genet. 1997 May;60(5):1059-67.
33. Begg CB. On the use of familial aggregation in population-based case probands for calculating penetrance J Natl Cancer Inst. 2002 Aug 21;94(16):1221-6.
34. Levy-Lahad E, Plon SE. Cancer. A risky business-assessing breast cancer risk. Science. 2003 Oct 24;302(5645):574-5.
35. Roukos DH, Kappas AM, Tsianos E. Role of surgery in the prophylaxis of hereditary cancer syndromes. Ann Surg Oncol. 2002 Aug-Sep;9(7):607-9. Editorial
36. Thompson D, Easton DF. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002;94:1358-1365.
37. Gruber SB, Petersen GM. Cancer risks in BRCA1 carriers: Time for the Next Generation of Studies. J Natl Cancer Inst 2002 Sept; 94(18): 1344-45, editorial.
38. Meijers-Heijboer H, van Geel B, van Putten WL, Henzen-Logmans SC, Seynaeve C, Menke-Pluymers MBE, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2001;345:159-64.
39. Gabriel SE, Woods JE, O'Fallon WM, Beard CM, Kurland LT, Melton LJ III. Complications leading to surgery after breast implantation. N Engl J Med 1997;336:677-682.
40. Guerra AB, Metzinger SE, Bidros RS, et al. Bilateral breast reconstruction with the deep inferior epigastric perforator (DIEP) flap: an experience with 280 flaps. Ann Plast Surg 2004 Mar;52(3):246-52.
41. Metcalfe KA, Esplen MJ, Goel V, Narod SA. Psychosocial functioning in women who have undergone bilateral prophylactic mastectomy. Psychooncology 2004 Jan;13(1):14-25.
42. King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA 2001 Nov 14;286(18):2251-6.
43. Jernstrom H, Lubinski J, Lynch HT, et al. Breast-feeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl cancer Inst 2004 Jul; 96(14): 1094-98.
44. Ries LAC, Eisner MP, Kasary CL., eds. SEER Cancer Statistics Review, 1973-1999 (National Cancer Institute, Bethesda, 2002).
45. Schrag D, Kuntz KM, Garber JE, Weeks JC. Decision analysis -- effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 and BRCA2 mutations. N Engl J Med 1997;336:1465-1471. [Erratum, N Engl J Med 1997;337:434.
46. Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst 1999;91:1475-1479.
47. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 2002;346:1616-1622.
48. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2002;346:1609-1615.
49. Garber JE, Hartman AR. Prophylactic oophorectomy and hormone replacement therapy: protection at what price? J Clin Oncol. 2004 Mar 15;22(6):978-80.
50. Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002 Jul 17;288(3):321-33.
51. Roukos DH, Kappas AM. Risks and benefits of risk-reducing surgery in inherited breast and gastric cancer susceptibility. Gastric Breast Cancer 2002; 1(3): 65-67.
52. Gswind A, Fischer OM, Ullrich A. The discovery of receptors tyrosine kinases: targets for cancer therapy. Nat Rev Cancer 2004; 4: 361-370.
53. van de Vijver MJ, He YD, van 't Veer LJ et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999-2009.
54. Roukos DH, Pavlidis N, Agnantis NJ. Gene-expression profile: the future in the outcome prediction and treatment of breast cancer.. Gastric Breast Cancer 2003;2:5- 8. (Accessed August 18, 2004 at http://www.gastriccancer.net/Gene-Expression_abs.asp ).
55. Klaren HM, van't Veer LJ, van Leeuwen FE, Rookus MA. Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation. J Natl Cancer Inst 2003; 95: 941-47.
56. Roukos DH, Agnantis NJ, Paraskevaidis E, Kappas AM. Challenges in surgical preventive decision for BRCA1/BRCA2-mutation carriers. Gastric Breast Cancer 2002; 1(3): 68-70.
57. Roukos DH, Agnanti NJ, Paraskevaidis E, Kappas AM. Approaching the dilemma between prophylactic bilateral mastectomy or oophorectomy for breast and ovarian cancer prevention in carriers of BRCA1 or BRCA2 mutations. Ann Surg Oncol. 2002 Dec;9(10):941-3.
58. Roukos DH. Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis-Invited Critique. Arch Surg 2003;138:1329.
59. Roukos DH, Kappas AM, Agnantis NJ. Perspectives and risks of breast-conservation therapy for breast cancer. Ann Surg Oncol. 2003 Aug;10(7):718-21. [editorial]
60. Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004 Jun 15;22(12):2328-35.
|



