Aiming at much better quality of life by similar mortality rates, research has been focused on developing of new techniques. EMR for early - stage esophagus, gastric and colorectal cancers, under strict control of several specific criteria, may replace conventional standard surgical treatment.
It is well recognized that early detection of gastrointestinal cancers is one of the most important factors that improves prognosis in these patients[2]. Newer techniques, such as magnifying endoscopy combined with chromoendoscopy [25,26,76], photodynamic diagnosis[77,78] and light-induced autofluorescence spectroscopy[79], to mention a few, hold promise for earlier detection of malignant lesions.
In the face of these newer implications, EMR emerges as an important new addition to our therapeutic armamentarium. It is expected to play an important role in establishing a diagnosis and treating early GI cancer in the future. Intensive research, newer technical implications and well-scheduled randomized trials, along with the adoption of common, universally-accepted criteria are needed in order to establish EMR as a first-line treatment or at least as a reliable alternative to surgical therapy for patients with early GI cancers
Endoscopic Mucosal Resection (EMR) is a relatively new therapeutic method for resection of elevated, flat, and depressed lesions throughout the gastrointestinal (GI) tract and has come to play an increasingly important role in the treatment of early gastrointestinal cancers and benign lesions (e.g. adenomas) throughout the gastrointestinal tract as well as sessile villous tumors of the colon. Furthermore, EMR is often applied in order to obtain specimens for accurate pathologic staging. The procedure was initially introduced by Japanese endoscopists as an alternative to traditional surgery and has lately gained favor in the West as a less invasive and equally effective method for removing certain neoplastic lesions in the GI tract, provided that specific indications are followed.[1-3]
In general, if a gastrointestinal lesion must be resected, open surgical operation, laparoscopy or endoscopic means may be used. Endoscopic methods can be grouped into two main technical categories: ablative techniques and EMR. There are several ablative techniques, among which electrocoagulation, argon plasma coagulation, laser photocoagulation, photodynamic therapy, ethanol injection therapy and cryotherapy are the most widely used.[2] The main drawback of the various ablative techniques is that, despite their relatively simple application and lack of associated complications, they do not allow a specimen to be obtained for further histopathologic evaluation. Therefore, such techniques are less desirable for the treatment of lesions thought to be malignant, premalignant or of uncertain etiology. In contrast, EMR techniques enable specimens' evaluation and help determine whether additional therapeutic intervention should take place depending on the depth of invasion and completeness of the resection.
Early cancers of the gastrointestinal tract are considered to have a generally good prognosis[4, 5] and complete cure is the therapeutic goal. Since lymph nodes harboring metastases cannot be reached endoscopically, EMR can only be applied to lesions with an extremely low risk of lymphatic metastasis. It has been conveyed that lymph node metastasis is related to a tumor'sinfiltration depth, size and differentiation grade in histological examination,[4-7] leading to the adoption of specific criteria for the application of EMR to ensure that it is reserved for localized lesions only. The exact determination of a tumor's depth of invasion is critical in assessing the results of studies and adopting certain indications.
Generally, early gastrointestinal cancer is divided into mucosal and submucosal, depending on whether or not the tumor has infiltrated the muscularis mucosa. Mucosal cancer is further subdivided into m1, which is carcinoma in situ, intraepithelial carcinoma or carcinoma with questionable invasion beyond the basement membrane; m2, when the cancer begins invading the lamina propria mucosa; and m3, which is cancer reaching the muscularis mucosa. Submucosal cancer is subdivided into sm1, when it infiltrates the upper third of the submucosa; sm2, when it involves the middle third as well; and sm3, when it infiltrates the lower third. [5,8]
Macroscopically, early gastrointestinal cancer is divided into tumors that are protruded (I), superficial (II) and excavated (III). Type II is further subdivided into elevated (IIa), flat (IIb) and depressed (IIc). In general terms, the elevation of type IIa is less than twice the thickness of the adjacent mucosa, whereas in type IIb, no elevation or depression can be observed, and in type IIc, the depression is only erosion.[4,9,10] Combinations of these types are common.
Technical aspects
The number of EMR techniques is constantly increasing and modifications of older techniques are frequently reported, in order to enhance its ease, safety and effectiveness. However, most of the various technical approaches follow some common principles. For instance, the first step of all techniques is to visualize the lesion, followed by an estimation of the possible depth of infiltration. Resection subsequently takes place and the specimens obtained are thoroughly examined histopathologically, in order to assess the completeness of the excision in depth and width.
2.1 Visualization
Regardless of the lesion's nature, it is important to accurately define its horizontal extent in order to resect it completely. Sometimes this can be difficult, especially when saline solution has been injected submucosally in order to lift the lesion as part of some resection techniques. Tissue staining can be accomplished with various stains according to the location and the nature of the lesion. Lugol solution, for example, at 2-2.5%, is mainly used in the esophagus to differentiate squamous dysplasia and carcinoma from normal mucosa, since lugol stains the glycogen of the normal squamous cells of the esophagus, while leaving the dysplastic or tumoral zones unstained. Indigo carmine 0.1-0.5% solution is frequently used to delineate stomach and colon lesions, as its blue color enhances mucosal contrast and allows better visualization of the lesion's borders and surface irregularities.[8,11-16] Methylene blue solution can be used to detect intestinal metaplasia (Barrett's esophagus).[17] Tissue staining (chromoendoscopy) is sometimes used along with magnifying endoscopy. In these cases crystal violet dye can be applied, as it stains the margins of the pits and offers a well-defined view of the lesion surface.[18] In order to further delineate the lesion, especially when a solution is injected submucosally, marking of its periphery is achieved by electrocautery with either a snares tip or a needle knife, depending on the resection technique applied.[19, 20]
2.2 Assessment of the tumor invasion depth
As stated previously, the depth of tumor invasion is highly correlated with the risk of lymph node metastasis. Therefore, it is useful to assess tumor infiltration depth before applying EMR, even though the pathologic evaluation of the specimen obtained after the procedure may help to determine the risk of lymph node metastasis more precisely. However, determination of the depth of tumor invasion is not reliably achieved by standard endoscopy, which is in this respect less useful in predicting lymph node infiltration.
In order to achieve this goal, various technological implications have emerged. Endoscopic Ultrasonography (EUS) is the modality most frequently used. The most commonly used instruments are 7.5 and 12 MHz probes, which are reported to have an overall accuracy of assessing (T stage) depth of penetration ranging from 71% to 92%.[2,21,22] Newer high-frequency probes (20-30 MHz) have better resolution and may show better results.[11,17] The combination of EUS with endoscopy is estimated to be more accurate in detecting submucosal invasion than endoscopy alone[23] and EUS is reported to compensate for the underestimation of lesions with submucosal invasion, which are endoscopically staged as mucosal cancer.[24] In order to increase the accuracy in depth invasion determination, magnifying endoscopy combined with chromoendoscopy,[15,25,26] as well as newer technologies, are being tested. For instance, Ultrasonic Tactile Sensor (UTS) provides a measurement of tissue stiffness,[27] whereas Laser-scanning Confocal Microscopy enables the achievement of an immediate serial virtual microscopic section through a fresh specimen (which has not actually been cut).[28] Finally, Optical Coherence Tomography (OCT) provides high resolution images of the mucosa and the submucosa based on the optical back scattering properties of tissues.[29, 30]
2.3 Resection techniques
EMR techniques are divided into two main categories: techniques "without suction" and those "with suction".
2.3.1. "Without suction" techniques
2.3.1.1. The Inject and cut technique
In 1973, Dehle et al. initially introduced the "inject and cut" technique, which is the oldest EMR technique, as a method for raising sessile polyps in the colon to facilitate snare resection. This method is solution also known in the West as saline-assisted, snare polypectomy.[2] Saline is submucosally injected by using a standard injection needle, after the periphery of the lesion has been outlined by electrocoagulation markings. The volume of saline used varies according to the lesion's size and location, and can range from as little as 5 ml in the esophagus to as much as 50 ml in the colon. After the lesion is lifted by the submucosal injection, it is captured by a snare, strangulated and resected by electrocautery. The specimen is then obtained endoscopically.[31] The snare used in the procedure usually has small hooks (shark-teeth snare) in order to better entrap the lesion and stop it from slipping.[17,32] There is a variety of solutions used for the submucosal injection apart from normal saline which diffuses quickly, such as diluted epinephrine solution,[33] sodium hyaluronate,[14,34] polyethylene glycol,[35] hydroxypropyl methyl cellulose36 and Glyceol (hypertonic solution of 10% glycerol, 5% fructose and saline solution).[37] However there is no consensus on the most effective solution and all of them appear to be safe. In order to better delineate the lesion, all solutions may be combined with chromoendoscopic dyes such as methylene blue.[2,31]
Appropriate submucosal injection is a key feature of the "lift and cut" technique, as it is for many other EMR techniques. That is because the injection enables complete and safe resection of the lesion, since it separates it from the muscularis propria, and therefore helps prevent perforation. (Figure) Another interesting feature of the submucosal injection is the so-called "no lifting sign". After submucosal injection of saline or other types of solutions, the lesion is supposed to lift and a bleb is formed. Lesions may not lift because of desmoplastic reaction, invasion from the lesion itself or submucosal fibrosis from prior biopsy, cautery or ulceration. It is not recommended to resect lesions that are not lifted easily due to the high probability of deep cancer invasion and the increased risk of perforation.[1,8,11,35]
2.3.1.2. The inject, lift and cut technique
This technique, which is also known as "strip biopsy," requires a double channel endoscope. Submucosal injection is performed in order to lift the lesion, as described previously. A snare and grasping forceps are advanced through the channels. The snare is placed around the lesion and the forceps are used to grasp and draw it towards the endoscope. The snare is then closed and the lesion is resected with electrocoagulation.[38,39] The main drawback of this technique is the need for a double channel endoscope, which is harder to manipulate, especially when the lesion is located tangentially to the endoscope.[37,40-42] Modifications of the "inject, lift and cut" technique have been reported, using, for instance, a partial transparent hood for lesions located tangentially to the endoscope41or using two small diameter endoscopes.[43]
2.3.1.3. The insulated tip diathermic knife technique (IT-EMR)
This technique is a modification of an older technique introduced by Hirao et al., using a diathermic endoscopic knife.[44] The IT-knife is a needle-knife with a ceramic ball attached to its tip in order to prevent electric leakage towards the deeper layers of the stomach and thus decrease the risk of perforation.
After the lesion is clearly visualized using indigo carmine and outlined using coagulation current, saline solution with diluted epinephrine is injected submucosally to separate the lesion from the muscularis propria. A 2mm wide incision is then made in the mucosa, using a conventional needle knife, to allow for the insertion of the IT-needle knife into the submucosa. Thereafter, the IT-knife is used to incise the mucosa circumferentially, just outside the marks, and the submucosal injection is repeated before snaring in order to avoid perforation. Following the injection, the lesion is snared with a standard snare and resected with blended electrosurgical current. The resected specimen is removed with grasping forceps.[20,45,46]
Other techniques using a small-caliber-tip transparent hood[34] or electrocautery incision forceps have been proposed for the resection of large superficial tumors.[14]
2.3.2. "With suction" techniques
2.3.2.1. Endoscopic Mucosal Resection using a cap-fitted endoscope (EMRC)
This technique was introduced by Inoue et a.l.[47] It requires a specialized transparent cap that is fitted to the tip of a standard endoscope and a small-diameter crescent-shaped snare. The cap presents an internal rim at the tip, in which the snare can be positioned. After the lesion is stained and outlined with the marks made at least 2mm from the lesion margin, diluted epinephrine or saline solution is injected submucosally. The total volume of the solution injected depends on the size of the lesion, yet it is important to inject enough to lift the whole lesion. The snare is then pre-looped along the rim of the EMRC cap: first, moderate suction is applied to normal mucosa to seal the cap outlet, and then, the snare is passed through the instrumental channel of the endoscope and is opened and fixed along the rim of the cap. The suction is released, the cap is subsequently used to take up the lesion completely, and the snare is closed, strangulating the sucked mucosa and creating a pseudopolyp, which is cut with electrocoagulation. The resected specimen is then removed by simply maintaining it within the cap. This technique can also be used for piece-meal resection of a lesion, and in this case, all the steps described should be repeated.[19] This technique is considered to be relatively simple and no additional training is needed for clinicians, as it is based on the widely used technique of endoscopic band ligation.[16]
2.3.2.2. Endoscopic Mucosal Resection with ligation (EMRL)
The EMRL technique uses a standard endoscopic variceal ligation device like the one reported initially by Stiegmann et al.,[48] i.e. one that is fitted on a single channel endoscope. After the lesion is stained and its periphery marked, saline solution is submucosally injected. The endoscope is then withdrawn and fitted with the ligation device, before it is reinserted. Afterwards, the lesion is aspirated into the hood of the device and an elastic band is applied in a manner similar to that employed with the banding of varices, creating an artificial polyp. If the lesion is not completely resected and a multiple-band ligation device is used, the procedure can be repeated. The endoscope is then withdrawn, the ligation device is disassembled and the endoscope is reinserted. The pseudopolyp is resected using a standard snare and electrocoagulation, either above or below the elastic band.[13,49] Some authors have reported EMRL without the use of submucosal injection,50 while others proposed the use of a "pneumoactivated" endoscopic variceal ligation device for EMRL, allowing the operational channel to be used for the insertion of the snare without prior disassembly of the ligation device.[12]
Other techniques using suction have also been reported, such as the endoscopic aspiration mucosectomy technique[37] and the Makuuchi tube method (used in the esophagus only).
The decision regarding which technique to use for each lesion encountered is not easy, since all techniques present limitations and difficulties. Not many comparisons of different techniques have been published until recently[38,39,42,50-52] and randomized studies did not include a large sample size of patients. Therefore, large, comparative, randomized, prospective trials are still awaited.
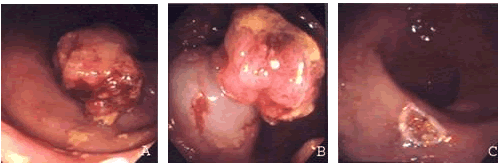
2.4. Procedures following EMR
The meticulous histopathological examination of the resected specimens and the precise report of the tumor's histology, infiltration depth and lymphatic or blood vessel involvement are extremely important in properly evaluating the results of the operation and determining whether an additional endoscopic or surgical intervention is needed. EMR is considered complete when both the horizontal and vertical margins are macroscopically and histopathologically free of neoplasia. However, many authors stress the need for a "security margin" (usually 2-3mm) between the malignancy and the margins of the specimen.[11,17,31] The need for additional treatment or surgery is determined by the fulfillment of the specific EMR indications used in each case.
EMR is mainly applied in the esophagus for superficial squamous carcinoma or severe dysplasia.[17,53] The main techniques used are EMRC, EMRL, the "inject lift and cut" technique and the EEMR-tube method.[11,5] Early esophageal cancer, by definition, is confined to the mucosa or submucosa regardless of any regional lymph node metastases.[35] The prognosis of early esophageal cancer after surgical treatment is estimated to be relatively good, with a 5-year survival rate exceeding 95% for stage 0 (Tis or m1) and 50-80% for T1 lesions.[22] The prognosis worsens as the tumor's infiltration depth increases. Japanese authors have reported disease-specific 5-year survival rates reaching 100% for cancer confined to the mucosa and significantly lower results for submucosal cancer, with 5-year survival rates ranging between 65% and 78%. The prognosis for submucosal cancers with lymph node metastasis is considered to be even poorer (27-43.6%).[5,7] Lymph node involvement is reported to be 0% for m1, 3.3% for m2, 12.2% for m3, 26.5% for sm1, 35.8% for sm2 and 45.9% for sm3 cancers.[5]
Surgery is the most frequently used treatment modality for esophageal cancer. However, the postoperative morbidity is reported to be 26-50% and postoperative mortality 3-12%.[22,54] It is therefore suggested that a less invasive technique like EMR could be preferable at least for m1 and m2 lesions, since in these cases, the risk of lymph node metastasis is low.
There is no consensus on the exact criteria for EMR to be applied in the esophagus,[5] but it is generally recommended for superficially, well- or moderately differentiated squamous carcinoma that is confined within the lamina propria. Some authors suggest that the lesion should be flat (IIa, IIb, IIc), less than 2cm in diameter and should not involve more than one third of the circumference of the esophageal wall.[1,8,40] The results of EMR procedures recorded so far are quite encouraging[1,19,55] and no significant differences are reported between EMR-treated and surgically treated patients that fit the above criteria.[5,56]
Recurrence after EMR is reported in 0-7.8% of cases. The risk of recurrence is higher in cases of piece-meal resection and in patients with multiple esophageal cancers.[53,56]
Complications recorded after EMR in the esophagus mainly consist of bleeding (usually minor), perforation and stenosis. Kodama and Kakegawa reported a 1.5% risk of bleeding, a 2.5% risk of perforation and a 2% risk of stenosis in a large review including more than 2400 patients. Piece-meal resection was associated with a slightly higher risk of perforation and stenosis compared to en bloc resection.5 Others presented similar results, and in most cases, no surgical intervention was needed.[16,31,56] The submucosal injection of sufficient saline volume is considered to be important in preventing perforation.[19]
Gastric adenomas, high-grade dysplasia and early gastric cancers are the main indications for applying EMR in the stomach.[12,31,45] The main techniques used are the "inject, lift and cut" technique, EMRC and EMRL.[11,33] Early gastric cancer (EGC) was defined by the Japanese Gastroenterological Endoscopy Society in 1962 and the Japanese Research Society for Gastric Cancer in 1963 as adenocarcinoma of the stomach confined to the mucosa or submucosa, irrespective of lymph node involvement.[4,57] The frequency of early gastric cancer has been increasing steadily in Japan during the last decades and now accounts for up to 50%[33] and in some institutions up to 60%[58] of all gastric cancers. This rise in the early detection of gastric cancer in Japan is followed by an increase in the proportion of EGC cases treated by EMR that now account for about 30-40% of all EGC cases.[46,58] The prognosis of EGC after surgery is quite favorable, with 5-year survival rates of greater than 90% being repeatedly reported in Japan, and more recently, in some centers in the West.[4,10,46,58] By definition, EGC presents only minor local invasion. However, lymph node invasion is not rare, ranging between 10-20% in many reports. The presence of nodal metastases is very closely associated with the depth of invasion, with mucosal cancers presenting node involvement in 0-7% (usually about 3%) of the cases and submucosal cancers in 15-30% (usually about 20%) of the cases.[4,11,17,35,40,46, 59] Furthermore, lymph node involvement increases with larger tumors6 and possibly poorly differentiated tumors, while other factors like lymphatic vessel involvement and female sex have also been reported to increase the risk.[4,60] It is estimated that the incidence of lymph node metastasis can be as low as 0.36% in patients having mucosal cancer with neither lymphatic vessel invasion nor tumor ulceration and a tumor diameter of less than 3cm.[46]
Surgical treatment is the established therapeutic option in gastric cancer. However, gastrectomy has a reported operative mortality rate of between 0.5 % and 6.5%.[35,46] Thus, surgical operation may be inappropriate for mucosal cancers since they may pose a lower risk of lymph node metastasis compared to the mortality rate of surgery. Furthermore, early cancers may not progress to advanced ones within the lifetime of the patient, since their doubling time ranges from two to ten years.[35,57]
The most widely used indications of EMR in the stomach are the ones accepted by the Japanese Gastroenterological Endoscopy Society, that include elevated-type intramucosal cancers less than 20mm in size, depressed-type mucosal cancers without ulceration less than 10mm in size, and intestinal type adenocarcinoma limited to the mucosa. However, studies using expanded indications also exist.
In an extensive Japanese review by Kojima et al., comprising more than 1800 patients, complete resection was carried out in 73.9% and en bloc resection was achieved in 75.8%. Other series, including some reviewed by Kojima, presented better results, achieving complete resection in [3-89% of the cases.[31,58] Lower rates of complete resection were achieved in patients treated using expanded indications.[58] When resection was incomplete, additional therapy consisted of surgery in 40% of the cases, while the rest were either re-treated endoscopically, mainly by EMR, laser therapy and ethanol injection plus heater-probe coagulation, or were simply observed. Recurrence was observed in 1.9% of tumors that were histopathologically documented as being eradicated. In another report by Ono et al., the recurrence rate was 18% in lesions whose complete resection was not confirmed.[46] In Kojima's review, only one patient was reported as dying of metastatic cancer and therefore the disease-specific survival rate was 99.05% in a follow-up period that ranged from 4 months to 11 years among different studies.[33]
Complications associated with EMR in the stomach are bleeding in 1.2-22% of the cases (depending on the definition of bleeding) and perforation in 0-5% of the procedures.[31,33,46]
|
EMR IN THE COLON AND RECTUM |
Adenomas (including those with severe dysplasia) and some early colorectal cancers are the main indications of EMR in the colon and rectum.[9,17] Some endoscopists see EMR as an improved technique of polypectomy.[17] However, it is additionally used for non-polypoid lesions, with the "inject and cut" and EMRC being the most frequently used techniques. [9,11,47] Early colorectal cancer is defined as malignancy limited to the submucosa. The macroscopic classification proposed by the Japanese endoscopists is similar to that used in other parts of the gastrointestinal tract (I, IIa, IIb, IIc, III), with the addition of an entity classified as Lateral Spreading Tumor (LST). LST is a tumor with predominant spread within the mucosa while still being relatively flat.[9]
The 5-year survival rate of early colonic cancer is in excess of 97% after surgical treatment. On the other hand, published 30-day post-operative mortality rates vary between 1% and 8%.[35] As in other parts of the gastrointestinal tract, the risk of lymph node involvement in early colorectal cancer increases in accordance with the depth of tumor infiltration. The risk of lymph node spread is reported to be on the order of 1% if the lesion is localized to the mucosa, and does not exceed 15% if it is confined to the submucosa.[11,35] The risk is also lower when the submucosal invasion is small (sm1) than when it is deep.[61] Nevertheless, it is not clear yet whether it is safe to perform EMR in lesions infiltrating the upper layer of the submucosa, since the risk of vascular invasion is still undetermined[8,17] and further prospective studies are needed. In the face of these uncertainties, no consensus has been reached on the exact criteria for EMR in the colon and rectum. Generally, EMR is used for well-differentiated adenocarcinoma of macroscopic type I (sessile), IIa, IIb, and IIc, which is not more than 1cm in diameter and is confined to the mucosa. Some authors report that this technique can also be used for Lateral Spreading Tumors (carpet-like lesions) since they are thought to rarely invade the submucosa despite their large size,[8,15,40] especially those with a granulonodular rather than flat surface.[18] Kudo reported in 1993 that among 674 cases of early colorectal cancer, 633 were treated endoscopically. Patients in ten of the cases had to undergo surgery, since the tumor was found to infiltrate deeply into the submucosa, while 74 others with submucosal invasion were followed-up. None of the patients treated only by endoscopy exhibited local recurrences, local lymph node metastases or liver metastases throughout their follow-up period, which was, however, not clearly described.[9] Other authors have reported complete resection in 86% to 97%[1] of lesions. Hotta et al. reported that the recurrence rate was higher in piecemeal resection than in en bloc resection.[62] Tanaka et al. treated [81] Lateral Spreading Tumors larger than 20 mm with EMR and reported a recurrence rate of 7.7% in patients who underwent follow-up without additional surgical treatment. The overall outcome of all patients was excellent.[18]
The main complications reported after EMR in the colon and rectum are bleeding in 1.4-16% of the patients and perforation in 0-1.2%.[1,18,35]
It is clear that many efforts have been made in order to make EMR as safe and feasible as possible. However some areas of uncertainty still remain.
One of the main controversial topics concerning EMR is the occurrence of synchronous and metachronous cancers throughout the gastrointestinal tract. Synchronous cancers in the esophagus were found in 22.3% of the patients in one report63 while metachronous cancers were found in 14.6% in another.64 Some authors suggest that the entire esophagus can be considered as a field of carcinogenesis.[64]
Similarly, in the stomach, synchronous cancers are found in 2-15%[4,33,46,58] of the cases and metachronous in 3-9%.[33,46,65,58] The occurrence of multiple synchronous lesions during the first endoscopy, advanced age and microsatellite instability are reported to increase the risk of metachronous cancer.[65,66] As mentioned in the esophagus, the hypothesis of "field carcinogenesis" is again proposed, in order to explain metachronous cancers.[4] In the face of these results, many authors stress the need for meticulous follow-up endoscopies in patients undergoing EMR in order to detect metachronous lesions and treat them at an early stage.[9,46, 64] This procedure seems reasonable, but close co-operation with the patient is necessary and this can be difficult in some occasions. The identification of high-risk patients can be helpful in deciding a more appropriate follow-up schedule and decreasing the overall cost of the treatment.
Another controversial issue concerning EMR procedures is the exact indications that are followed. As already mentioned, there is no consensus on the exact criteria followed for EMR, especially in the esophagus and colon. Many authors advocate the need for an expansion of the usual criteria and studies are taking place following criteria different from those usually adopted.[7,20,33,46] Nevertheless, in order to compare the results coming from different researchers and create a better picture concerning EMR procedures, shared, universally accepted indications are needed. Differences between Japanese and Western histopathologic classification systems used to describe early forms of GI cancers further complicate the correct interpretation of the Japanese experience by Western endoscopists. Efforts targeting the adoption of common classification systems must be continued.[67,68]
In addition, large, prospective, randomized trials should take place in order to directly compare surgery using each respective EMR techniques and extrapolate solid evidence on when and how EMR should be performed. This is of great importance, considering the fact that most reports concerning EMR results focus on lymph node status, not taking into account the haematogenous spread that occurs independently of lymphogenous involvement, especially in the esophagus.[69]
Furthermore, additional studies are needed, in order to determine the appropriate treatment of residual lesions or local recurrences after EMR. At present there is no consensus and many different treatment strategies are applied in the various studies, with the most frequently used treatment modalities being surgery, EMR and ablative techniques[33,70] Nevertheless, there are no large randomized trials directly comparing these strategies and it is not clear what should be done in cases of incomplete resection or recurrence.
At the same time, newer potential EMR indications are being proposed and need further research in order to become established. Among them, EMR in Barrett's esophagus[32,55,71,72] and EMR in the treatment of carcinoid tumors[73,74] are reported with increasing frequency.
Barrett's esophagus is rarely reported in the Japanese series. Therefore, the main interest in EMR for this condition comes from Western endoscopists. It is stated that esophageal adenocarcinoma develops in approximately 0.5% of patients with Barrett's esophagus per year.[75] Low-grade dysplasia is reported to progress to high-grade dysplasia or adenocarcinoma within 5 years in 10-28% of the patients. It is, however, meaningful to stress that by the time biopsy specimens show high-grade dysplasia, approximately one-third of patients already have an invasive cancer. Therefore, current guidelines suggest esophagectomy or intensive endoscopic surveillance for patients with high-grade dysplasia within Barrett's esophagus.[54] In the face of the high mortality and morbidity associated with esophagectomy, it is proposed that EMR could be used for both diagnostic and therapeutic reasons, alone or in conjunction with ablative techniques.[32,55,71,72] The results of some preliminary studies are encouraging but more reports are needed and long-term results are awaited in order to accurately determine the role of EMR in Barrett's esophagus.
It is well recognized that early detection of gastrointestinal cancers is one of the most important factors that improves prognosis in these patients.[2] Newer techniques, such as magnifying endoscopy combined with chromoendoscopy,[25,26,76] photodynamic diagnosis[77,78] and light-induced autofluorescence spectroscopy,79 to mention a few, hold promise for earlier detection of malignant lesions.
In the face of these newer implications, EMR emerges as an important new addition to our therapeutic armamentarium. It is expected to play an important role in establishing a diagnosis and treating early GI cancer in the future. Intensive research, newer technical implications and well-scheduled randomized trials, along with the adoption of common, universally-accepted criteria are needed in order to establish EMR as a first-line treatment or at least as a reliable alternative to surgical therapy for patients with early GI cancers.
1. American Society for Gastrointestinal Endoscopy. Technology status evaluation report. Endoscopic Mucosal Resection. Gastrointestinal Endoscopy 2000; 52:860-863.
2. Fleischer D. Endoscopic mucosal resection: (not) made in the USA (so commonly). A dissection of the definition, technique, use, and controversies. Gastrointestinal Endoscopy 2000; 52:440-444.
3. Aabakken L. Endoscopic Tumor Diagnosis and Treatment. Endoscopy 2003; 35:887-890.
4. Everett SM, Axon ATR. Early gastric cancer in Europe. Gut 1997; 41:142-150.
5. Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: A summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery 1998; 123:432-439.
6. Nakamura K, Morisaki T, Sugitani A, et al. An Early Gastric Carcinoma Treatment Strategy Based on Analysis of Lymph Node Metastasis. Cancer 1999; 85:1500-1505.
7. Tajima Y, Nakanishi Y, Ochiai A, et al. Histopathologic Findings Predicting Lymph Node Metastasis and Prognosis of Patients with Superficial Esophageal Carcinoma. Cancer 2000; 88:1285-1293.
8. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointestinal Endoscopy 2003; 57:567-579.
9. Kudo S-e. Endoscopic Mucosal Resection of Flat and Depressed Types of Early Colorectal Cancer. Endoscopy 1993; 25:455-461.
10. Ohta H, Noguchi Y, Takagi K, Nishi M, Kajitani T, Kato Y. Early Gastric Carcinoma With Special Reference to Macroscopic Classification. Cancer 1987; 60:1099-1106.
11. Greff M, Palazzo L, Ponchon T, Canard JM, and the Council of the French Society of Gastrointestinal Endoscopy. Guidelines of the French Society of Digestive Endoscopy: Endoscopic Mucosectomy. Endoscopy 2001; 33:187-190.
12. Akiyama M, Ota M, Nakajma H, Yamagata K, Munukata A. Endoscopic mucosal resection of gastric neoplasms using a ligating device. Gastrointestinal Endoscopy 1997; 45:182-186.
13. Sakai P, Filho FM, Iryia K, et al. An endoscopic technique for resection of small gastrointestinal carcinomas. Gastrointestinal Endoscopy 1996; 44:65-68.
14. Yamamoto H, Sekine Y, Higashizawa T, et al. Successful en bloc resection of a large superficial gastric cancer by using sodium hyaluronate and electrocautery incision forceps. Gastrointestinal Endoscopy 2001; 54:629-632.
15. Kudo S-e, Kashida H, Tamura T, et al. Colonoscopic Diagnosis and Management of Nonpolypoids Early Colorectal Cancer. World Journal of Surgery 2000; 24:1081-1090.
16. Inoue H. Endoscopic mucosal resection for esophageal and gastric cancers. Canadian Journal of Gastroenterology 1998; 12:355-359.
17. Ponchon T. Endoscopic Mucosal Resection. Journal of Clinical Gastroenterology 2001; 32:6-10.
18. Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointestinal Endoscopy 2001; 54:62-66.
19. Inoue H, Kawano T, Tani M, Takeshita K, Iwai T. Endoscopic mucosal resection using a cup: Techniques for use and preventing perforation. Canadian Journal of Gastroenterology 1999; 13:477-480.
20. Ohkuwa M, Hasokawa K, Boku N, Ohtu A, Tajiri S, Yoshida S. New Endoscopic Treatment for Intramucosal Gastric Tumors Using an Insulated-Tip Diathermic Knife. Endoscopy 2001; 33:221-226.
21. Harada N, Hamada S, Kubo H, et al. Preoperative Evaluation of Submucosal Invasive Colorectal Cancer Using a 15-MHz Ultrasound Miniprobe. Endoscopy 2001; 33:237-240.
22. Enzinger PC, Mayer RJ. Esophageal Cancer. The New England Journal of Medicine 2003; 349:2241-2252.
23. Ohashi S, Segawa K, Okamura S, et al. The utility of endoscopic ultrasonography and endoscopy in the endoscopic mucosal resection of early gastric cancer. Gut 1999; 45:599-604.
24. Yanai H, Noguchi T, Mizumachi S, et al. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut 1999; 44:361-365.
25. Kato S, Fujii T, Koba I, et al. Assesment of Colorectal Lesions Using Magnifying Colonoscopy and Mucosal Dye Spraying: Can Significant Lesions Be Distinguished? Endoscopy 2001; 33:306-310.
26. Kudo S-e, Tamura S, Nakajima T, Yamano H-o, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointestinal Endoscopy 1996; 44:8-14.
27. Yoshida T, Inoue H, Kawano T, Takeshita K, Iwai T. The Ultrasonic Tactile Sensor: In Vivo Clinical Application for Evaluation of Depth of Invasion in Esophageal Squamous Cell Carcinomas. Endoscopy 1999; 31:442-446.
28. Inoue H, Igari T, Nishikage T, Ami K, Yoshida T, Iwai T. A Novel Method of Virtual Histopathology Using Laser-Scanning Confocal Microscopy In-Vitro with Untreated Fresh Specimens from the Gastrointestinal Mucosa. Endoscopy 2000; 32:439-443.
29. Sivak MVJ, Kobayashi K, Izatt JA, et al. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointestinal Endoscopy 2000; 51:474-479.
30. Das A, Sivak MVJ, Chak A, et al. High-resolution endoscopic imaging of the GI tract: a comparative study of optical coherence tomography versus high-frequency catheter probe EUS. Gastrointestinal Endoscopy 2001; 54:219-224.
31. Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointestinal Endoscopy 2002; 55:390-396.
32. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointestinal Endoscopy 2000; 52:328-332.
33. Kojima T, Parra-Blanco A, Takahashi H, Rikiya F. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointestinal Endoscopy 1998; 48:550-555.
34. Yamamoto H, Kawata H, Sunada K, et al. Successful En-Bloc Resection of Large Superficial Tumors in the Stomach and Colon Using Sodium Hyaluronate and Small-Caliber-Tip Transparent Hood. Endoscopy 2003; 35:690-694.
35. Rembacken BJ, Gotoda T, Fujii T, Axon ATR. Endoscopic Mucosal Resection. Endoscopy 2001; 33:709-718.
36. Feitoza AB, Gostout CJ, Burgart LJ, Burkert A, Herman LJ, Rajan E. Hydroxypropyl methylcellulose: a better submucosal fluid cushion for endoscopic mucosal resection. Gastrointestinal Endoscopy 2003; 57:41-47.
37. Torii A, Sakai M, Kajiyama T, et al. Endoscopic aspiration mucosectomy as curative endoscopic surgery: Analysis of 24 cases of early gastric cancer. Gastrointestinal Endoscopy 1995; 42:475-479.
38. Kajiyama T, Hajiro K, Sakai M, et al. Endoscopic resection of gastrointestinal submucosal lesions: a comparison between strip biopsi and aspiration lumpectomy. Gastrointestinal Endoscopy 1996; 44:404-410.
39. Tanabe S, Koizumi W, Kokutou M, et al. Usefulness of endoscopic aspiration mucosectomy as compared with strip biopsy for the treatment of gastric mucosal cancer. Gastrointestinal Endoscopy 1999; 50:819-822.
40. Shim C-S. Endoscopic Mucosal Resection: An Overview of the Value of Different Techniques. Endoscopy 2001; 33:271-275.
41. Noda M, Kobayashi N, Kanemasa H, et al. Endoscopic mucosal resection using a partial transparent hood for lesions located tangentially to the endoscope. Gastrointestinal Endoscopy 2000; 51:338-343.
42. Matsusita M, Hajiro K, Okazaki K, Takakuwa H. Endoscopic mucosal resection of gastric tumors located in the lesser curvature of the upper third of the stomach. Gastrointestinal Endoscopy 1997; 45:512-515.
43. Takechi K, Mihara M, Saito Y, et al. A modified technique for endoscopic mucosal resection of small gastric carcinomas. Endoscopy 1992; 24:229-231.
44. Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointestinal Endoscopy 1988; 34:264-269.
45. Miyamoto Si, Muto M, Hamamoto Y, et al. A new technique for endoscopic mucosal resection with an insulate-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointestinal Endoscopy 2002; 55:576-581.
46. Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001; 48:225-229.
47. Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach and colon mucosal lesions. Gastrointestinal Endoscopy 1993; 39:58-62.
48. Stiegmann G, Cambre T, Sun J. A new endoscopic elastic band ligating device. Gastrointestinal Endoscopy 1986; 32:230-233.
49. Fleischer D, Wang GQ, Dawsey S, et al. Tissue band ligation followed by snare resection (band and snare): a new technique for tissue acquisition in the esophagus. Gastrointestinal Endoscopy 1996; 44:68-72.
50. May A, Gossner L, Bahrens A, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointestinal Endoscopy 2003; 58:167-175.
51. Ryu C, Kim H, Jang J, et al. The comparison of variable methods of endoscopic mucosal resection for early gastric cancer and gastric flat adenoma including insulated-tip electrosurgical knife EMR. Gastrointestinal Endoscopy 2003; 57:AB186.
52. Nagai T, Torishima R, Nakashima H, et al. Saline-Assisted Endoscopic Resection of Rectal Carcinoids: cap Aspiration Method Versus Simple Snare Resection. Endoscopy 2004; 36:202-205.
53. Nomura T, Boku N, Ohtsu A, et al. Recurrence After Endoscopic Mucosal Resection for Superficial Esophageal Cancer. Endoscopy 2000; 32:277-280.
54. Spechler SJ. Barrett's Esophagus. The New England Journal of Medicine 2002; 346:836-842.
55. 55. Ell C, May A, Gossner L, et al. Endoscopic Mucosal Resection of Early Cancer and High-Grade Dysplasia in Barrett's Esophagus. Gastroenterology 2000; 118:670-677.
56. Fujita H, Sueyoshi S, Yamana H, et al. Optimum Treatment Strategy for Superficial Esophageal Cancer: Endoscopic mucosal Resection versus Radical Esophagectomy. World Journal of Surgery 2001; 25:424-431.
57. Shimizu S, Tada M, Kawai K. Early Gastric Cancer: Its Surveillance and Natural Course. Endoscopy 1995; 27:27-31.
58. Takekoshi T, Baba Y, Ota H, et al. Endoscopic Resection of Early Gastric Carcinoma: Results of a Retrospective Analysis of 308 Cases. Endoscopy 1994; 26:352-358.
59. Hiki Y, Shimao H, Mieno H, Sakakibara Y, Kobayashi N, Saigenji K. Modified Treatmnt of Early Gastric Cancer: Evaluation of Endoscopic Treament of Early Gastric Cancers with Respect to Treatment Indication Groups. World Journal of Surgery 1995; 19:517-522.
60. Abe N, Watanabe T, Suzuki K, et al. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. The American Journal of Surgery 2002; 183:168-172.
61. Kikuchi R, Takano M, Takagi K, et al. Management of Early Invasive Colorectal CancerRisk of Recurrence and Clinical Guidelines. Diseases of the colon and rectum 1995; 38:1286-1295.
62. Hotta K, Fujii T, Ono A, et al. Local recurrence after endoscopic treatment for the colorectal tumors:Is it necessary to resect in one piece? Gastrointestinal Endoscopy 2003; 57:AB 222.
63. Morita M, Kuwano H, Yasuda M, et al. The Multicentric Occurrence of Squamous Epithelial Dysplasia and Squamous Cell Carcinoma in the Esophagus. Cancer 1994; 74:2889-2895.
64. Shimizu Y, Tukagoshi H, Masahiro F, Hosokawa M, Kato M, Asaka M. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointestinal Endoscopy 2001; 54:190-194.
65. Arima N, Adachi K, Katsube T, et al. Predictive Factors for Metachronous Recurrence of Early Gastric Cancer After Endoscopic Treatment. Journal of Clinical Gastroenterology 1999; 29:44-47.
66. Kawamura A, Adachi K, Ishihara S, et al. Correlation between Microsatellite Instability and Metachronous Disease Recurrence after Endoscopic Mucosal Resection in Patients with Early Stage Gastric Carcinoma. Cancer 2001; 91:339-345.
67. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47:251-255.
68. Willis J, Riddell RH. Biology versus terminology: East meets West in surgical pathology. Gastrointestinal Endoscopy 2003; 57:369-376.
69. O'Sullivan GC, Shanahan F. Esophagogastric Cancer-Time to change the paradigm. The American Journal of Gastroenterology 2000; 95:2153-2154.
70. Brooker JC, Saunders BP, Shah SG, Thapar CJ, Suzuki N, Williams CB. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointestinal Endoscopy 2002; 55:371-375.
71. May A, Gossner L, Pech O, et al. Intraepithelial High-Grade Neoplasia and Early Adenocarcinoma in Short-Segment Barrett's Esophagus (SSBE): Curative Treatment Using Local Endoscopic Treatment Techniques. Endoscopy 2002; 34:604-610.
72. Seewald S, Akaraviputh T, Seitz U, et al. Circumferential EMR and complete removal of Barrett's epithelium:a new approach to management of Barrett's esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointestinal Endoscopy 2003; 57:854-859.
73. Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic Mucosal Resection in the Management of Gastric Carcinoid Tumors. Endoscopy 2003; 35:203-206.
74. Okamoto Y, Fujii M, Tateiwa S, et al. Treatment of Multiple Rectal Carcinoids by Endoscopic Mucosal Resection Using a Device for Esophageal Variceal Ligation. Endoscopy 2004; 36:469-470.
75. Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is There Publication Bias in the Reporting of Cancer Risk in Barrett's Esophagus? Gastroenterology 2000; 119:333-338.
76. Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. The New England Journal of Medicine 1998; 339:1277-1284.
77. Holstein CSv, Nilsson AMK, Anderson-Engels S, Willen R, Walther B, Svanberg K. Detection of adenocarcinoma in Barrett's oesophagus by means of laser induced fluorescence. Gut 1996; 39:711-716.
78. Mayinger B, Neidhardt S, Reh H, Martus P, Hahn EG. Fluorescence induced with 5-aminolevulinic acid for the endoscopic detection and follow-up of esophageal lesions. Gastrointestinal Endoscopy 2001; 54:572-578.
79. Mayinger B, Horner P, Jordan M, et al. Light-induced autofluorescence spectroscopy for tissue diagnosis of GI lesions. Gastrointestinal Endoscopy 2000; 52:395-400.
|


