
Current
best practices and rationalistic perspectives in causation-based
prevention, early detection and multidisciplinary treatment
of breast and gastric cancer
|
|
Volume 2 - Number 1 - Jan/May 2003 |
|
COMMENTARY |
|
Breast-Conserving Surgery and Risk of Positive Margins in Breast Cancer Dimitrios H. Roukos, MD, Niki J. Agnantis, MD, Haralambos Batsis, MD, Evangelos Paraskevaidis, MD and Angelos M. Kappas From the Department of Surgery, (DHR, HB, AMK), Department of Pathology (NJA), and Department of Obstetrics and Gynecology (EP). All at Ioannina University School of Medicine, Ioannina, 451 10 Ioannina, Greece, droukos@cc.uoi.gr Recent studies with long-term follow-up establish that overall and breast cancer-specific survival do not differ after breast conservation treatment or total mastectomy. These reports will undoubtedly lead to an increasing rate of breast-conserving surgery replacing steadily total mastectomy. What is the risk of this expected trend especially on close or positive tumor margin and its impact on local recurrence? Is the current adjuvant treatment –radiation, chemotherapy, tamoxifen-so effective that can replace a standard surgical re-excision in cases with close or positive margins? Is local failure in the conserved breast a true recurrence or a new primary breast tumor? |
|
Complete removal of the primary tumor by surgery –curative or R0 resection in the AJCC/UICC-TNM classification1- has been established standard in the surgical management of solid tumors. When the disease is identified at an early-stage cancer, this principal goal of surgery for an R0 resection is achievable by a less extensive surgery. This creative thoughtful concept represents an important advance towards a patient’s lower morbidity and better quality of life. A tumor stage-stratified treatment has long been considered for breast cancer[2] and is recently also suggesting for other solid tumors including gastric cancer.[3,4] |
|
However, precondition for a wide clinical implication of the limited surgical approach is the availability of long-term survival data from randomized controlled trials (RCTs) that provide scientific evidence supporting a lower side-effects profile of this strategy, as compared with extensive surgery without any increase in the risk of treatment failure and death. A trend towards a less extensive surgery has already been started for the most common cancer sites such the prostate, and gastrointestinal tract and the treatment effect is now evaluating in ongoing RCTs. |
|
For breast cancer however, there are now accumulating evidence-based data that allow us to drawn conclusions about the effectiveness of breast conservation therapy. Survival data from RCTs after a follow-up of 5 or 10 years[5-11] showed no significant difference in overall survival or breast-cancer specific survival after breast-conserving surgery or mastectomy. The lack of long-term follow-up data was an argument for caution of breast-conserving surgery because of the long natural history of breast cancer. Now, the recently published 20-years results of the two landmark studies by Fisher et al[12] and Veronesi et al.[13] confirm that survival does not differ between the two procedures. |
|
Breast conservation therapy, as a patient’s friendly and preferable procedure that leads to a better quality of life than total mastectomy, is increasingly accepting and will be widely used in the next years. However, this widespread clinical use raises several key questions: (a) Does the trend and efforts towards a steadily increasing number of patients who treated with a less extensive surgery, by expanding the eligibility criteria, increase the risk of close or positive tumor margin? (b) what is the effect of microscopic evidence of positive margin on the risk of ipsilateral breast tumor recurrence (IBTR)? (c) should the women with a final close or positive resection margin undergone further surgery or a re-excision can be avoided by the availability of current effective adjuvant treatment –radiation, chemotherapy, tamoxifen-? (d) Is local failure a true recurrence or a new primary ipsilateral breast tumor ? |
|
Data from randomized and nonrandomized studies allow us today to a scientific approach of these questions. The answer to the first question is clearly yes. The rate of positive margin on the final excision is high and ranges from 10%[12] to 48%[10] in RCTs and from 22%[14] to 41% [15] overall rate of close (<2mm) or positive margin in recent reports of nonrandomized studies. This widespread variation is attributable to the selection criteria, definition of margin status, extent of conservative surgery (lumpectomy, quadrantectomy, local/wide excision), tumor size, adjuvant treatment and institution. |
|
Margin status and a number of several other clinical and pathologic factors that include young patient age, extensive intraductal component (EIC), histologic type and grade, lymph-vascular invasion and the presence of ductal carcinoma in situ (DCIS), have been assessed for their ability to predict an increased risk of IBTR. |
|
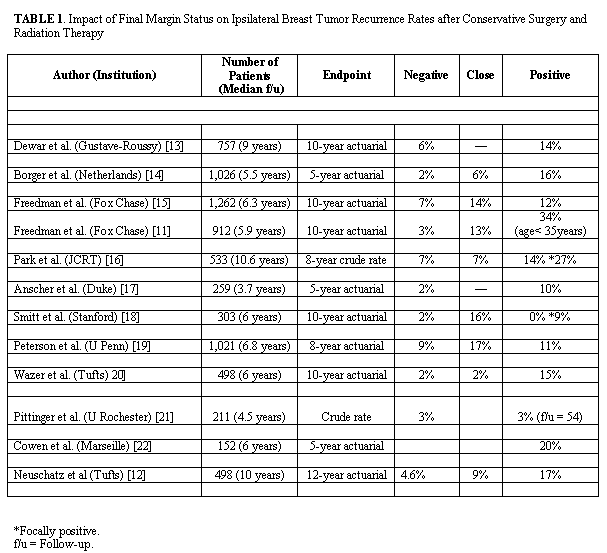
Positive margin on the final resection specimens seems to be associated with increased risk of local failure. Long-term data on the use of breast-conserving therapy in patients with positive margins is limited and recurrence rate varies considerably. In most series the risk of IBTR after breast–conserving and radiation has been shown to be two to four times greater in the presence of a positive or close (< 2mm) margin compared with negative margins[14-25] (Table 1). The highest risk of recurrence has been observed in certain subgroups. In young women aged <35 or <45 years with positive both EIC-tumors and margin a high risk of 34%[14] and 55%[26] has been reported. These reports provide some evidence that the increased risk of IBTR in young women may be attributed to an association of young age with EIC positivity and close or positive resection margins.[27] However, no significant increase in the rate of IBTR has been found in other reports.[14,22,24] The variation in these results may be related to the extent of the surgical resection for the primary tumor, the presence or absence of an EIC, the definition of a positive margin, the number of margins that are positive, the extent of the margin positivity and the use of adjuvant chemotherapy and/or tamoxifen. |
|
The extent of conservative surgery may influence the risk of local recurrence. In the Milan II trial,[28] the breast recurrence rate for patients with positive margins was 12% for those undergoing a quadrantectomy compared with 17% for those whose primary surgical procedure was lumpectomy. In the study by Silberstein at al. a wider margin excision of normal breast tissue surrounding ductal carcinoma in situ (DCIS) had resulted in fewer ipsilateral recurrences.[29] Although additional experience is needed to confirm the association of close or positive margins and increase risk of recurrence, negative margin continues to remain the cornerstone in the breast-conserving therapy. |
|
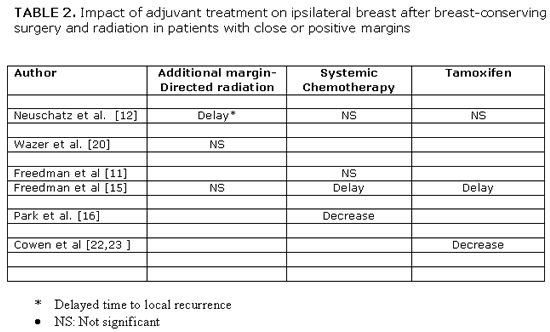
Clinically important is the answer to the third question about the effect of adjuvant treatment on IBTR in women with positive margins. If current adjuvant treatment, that includes more aggressive margin-directed radiation dose escalation, systemic chemotherapy and/or tamoxifen, would be so effective to replace surgical re-excision without increasing risk of local recurrence, it could result in a wider use of breast-conserving therapy. However, data addressing this question are limited and of low level (II-IV) of evidence. Table 2 summarizes the treatment effect of adjuvant treatment in women with close or positive margins.[14,15,18,19,23,25,26] Aggressive radiation dose escalation to the tumor bed, delayed time to local recurrence up to the first 5 years after breast conservation treatment in women with close or focally positive margin but had no significant decrease in the ultimate 10-year cumulative incidence of IBTR.[15,18,23] |
|
A similar treatment effect has been reported by the use of systemic adjuvant chemotherapy or tamoxifen. The use of adjuvant chemotherapy in women with focally positive margins reduced the rate of IBTR from 18% without chemotherapy to 7% with chemotherapy at 8 years in the report by Park et al,[19] but in other studies this systemic treatment either had no effect on IBTR[14,15] or delayed time to local recurrence in the first 5 years without a significant reduction in the ultimate 10-year local recurrence rate.[18] |
|
Tamoxifen has resulted in a delay in IBTR up to a median of 6.7 years in the report by Freedman et al.[15] Cowen et al.[25,28] also reported that adjuvant hormone use increased the local recurrence free survival with positive margins but not with negative margins up to 10 years after breast-conserving surgery and radiation without chemotherapy. All these retrospective studies are limited by the small number of patients, interactions between final margin status and young age, EIC or other prognostic factors and the absence of multivariate analysis. The contracting results therefore do not surprise and do not allow us to drawn conclusions. There is some suggestion that adjuvant treatment may delay time to recurrence but is unable to reduce the incidence of IBTR in a time period longer than 5 years after breast conserving treatment in patients with close or positive margins. The available data cannot provide any evidence that adjuvant treatment can replace surgical re-excision in women with close or positive margins in final specimen after breast-conserving surgery. Surgical re-excision of positive or close margins continues to remain the standard procedure despite the availability of effective adjuvant treatment .[14,15] |
|
Is local failure after breast conservation a true recurrence or a new primary tumor? The answer is important for decision-making about diagnostic and treatment. True recurrence at the tumor bed suggests the need for more aggressive local treatment, whereas tumor reappearance at a remote site may be prevented by total mastectomy. |
|
Site and time –early or late- of recurrence help in the understanding of tumor nature. Based on the site of recurrence, Neuschatz et al. separated local failure into central/peripheral and remote recurrence. They found that 70% of women with central/peripheral recurrence had initial margins that were close or positive, whereas only 46% of the remote recurrences were in this category. In the entire cohort, 41% had close or positive margins and the authors believe that the remote recurrences can be viewed as a crossection of the initial cohort.[12] Long-term follow-up studies in the total of women undergoing breast-conserving treatment consistently indicate that most early (< 5 years) failures are true recurrences, whereas most late (> 5- or 10 years) recurrences are new primaries.[13,15,30,31] These data also indicate that irradiation of the whole breast does not provide full long-term protection against local recurrence. |
|
Late appearance of malignancy at a remote site either it is originated from a small foci of carcinoma undetected at initial diagnosis[35-37] or it is a new primary can be prevented by total mastectomy. However, the key question is how can be identified before treatment these women who will develop a late recurrence. |
|
MCM Outlet Scientific evidence for the important role of clear surgical margins not only for local tumor control but also for the combat of lymphatic spread and lymph node metastases is recently provided by relevant basic research. Padera et al.[35] found in mice that functional lymphatic vessels in the tumor margin are sufficient for lymphatic metastasis and confirmed this observation in patients with lung cancer. Therefore the authors suggest that the tumor margins should be treated aggressively by local treatment, such as surgery and radiation, to combat lymphatic dissemination. |
|
Breast conservation therapy is the treatment of choice, provided that the margins of resected specimens are free of tumor, point out even the strong supporters of this treatment option.[12] It is clear that we need new markers that can define who individual woman needs a less or more extensive surgery to prevent local failures. Promises for incorporation of such new biologic predictors into clinical practice are provided by recent studies which use DNA-microarrays gene-expression data and cyclin E-levels.[36-39] There is hope that these new markers will facilitate an appropriate surgical decision-making between breast-conserving surgery and total mastectomy. |
Conclusion |
|
Surgery with clear resection margins remains the principal goal of the breast-conserving therapy. Data about the ability of current adjuvant treatment to replace surgical re-excision in close or positive margins are limited and of low level of evidence (II-IV). Studies with the newer more effective chemotherapeutic agents are needed but until then re-excision remains standard. |
|
References |
|
1. Cancer Staging Manual, sixth edition of the American Joint Committee on Cancer (AJCC). 2003 (www.cancerstaging.org). 2. Fisher B. Breast-cancer management: alternatives to radical mastectomy. N Engl J Med 1979;301:326-328. 3. Elisaf M., Roukos DH, Briasoulis E, Michos KM, Agnantis NJ, Kappas AM. Comparing recent advances in gastric and breast cancer. Gastric Breast Cancer 2002; 1(3): 59-64. 4. Roukos DH. Tumor stage-based tailored therapeutic strategy for gastric cancer: rational approach or new trend? Gastric Breast Cancer 2003; 2(1): 1-4. 5. Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med 1981;305:6-11. 6. Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665-673. 7. Sarrazin D, Le MG, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol 1989;14:177-184. 8. Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. In: Consensus development conference on the treatment of early-stage breast cancer. Journal of the National Cancer Institute monographs. No. 11. Bethesda, Md.: National Cancer Institute, 1992:19-25. (NIH publication no. 90-3187.) 9. Arriagada R, Le MG, Rochard F, et al. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol 1996;14:1558–1564. 10. van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-1150. 11. Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995;332:907-911. 12. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-1241. 13. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-1232 14. Freedman GM, Hanlon AL, Fowble BL, Anderson PR, Nikolaou N. Recursive partitioning identifies patients at high and low risk for ipsilateral tumor recurrence after breast-conserving surgery and radiation. J Clin Oncol 2002; 20: 4015-21. 15. Neuschatz AC, DiPetrillo T, Safaii H, et al. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer 2003;97:30-39. 16. Dewar JA, Arriagada R, Benhamou S, et al. Local relapse and contralateral tumor rates in patients with breast cancer treated with conservative surgery and radiotherapy (Institute Gustave Roussy 1970-1982). IGR Breast Cancer Group. Cancer 1995;76:2260–2265. 17. Borger J, Kemperman H, Hart A, et al. Risk factors in breast conservation therapy. J Clin Oncol 1994;12:653–660. 18. Freedman G, Fowble B, Hanlon A, et al. Patients with early-stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys 1999;44:1005–1015. 19. Park C, Mitsumori M, Recht A, et al. The relationship between pathologic margin status and outcome after breast-conserving therapy. Int J Radiat Oncol Biol Phys 1998;42:125. 20. Anscher MS, Jones P, Prosnitz LR, et al. Local failure and margin status in early-stage breast carcinoma treated with conservation surgery and radiation therapy. Ann Surg 1993;218:22–28. 21. Smitt MC, Nowels KW, Zdeblich MJ, et al. The important of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer 1995;76:259–267. 22. Peterson ME, Schultz DJ, Reynolds C, et al. Outcomes in breast cancer patients relative to margin status after treatment with breast-conserving surgery and radiation therapy: The University of Pennsylvania experience. Int J Radiat Oncol Biol Phys 1999;43:1029–1035 23. Wazer DE, Schmidt-Ullrich RK, Ruthazer R, et al. Factors determining outcome for breast-conserving irradiation with margin-directed dose escalation to the tumor bed. Int J Radiat Oncol Biol Phys 1998;40:851–858. 24. Pittinger TP, Maronian NC, Poulter CA, et al. Importance of margins status in outcome of breast-conserving surgery for carcinoma. Surgery 1994;116:605–608. 25. Cowen D, Largillier R, Bardou VJ, et al. Positive margins after conservative treatments impacts local control and possibly survival in node-negative breast cancer. Int J Radiat Oncol Biol Phys 1998;42:126. 26. Cowen D, Houvenaeghel G, Bardou V-J, et al: Local and distant failures after limited surgery with positive margins and radiotherapy for node-negative breast cancer. Int J Radiat Oncol Biol Phys 47: 305-312, 2000 27. Kurtz JM, Jacquemier J, Amalric R, et al: Why are local recurrences after breast-conserving therapy more frequent in younger patients? J Clin Oncol 8: 591-598, 1990 28. Veronesi U, Luini A, Galimberti V, et al. Conservation approaches for the management of Stage I/II carcinoma of the breast: Milan Cancer Institute trials. World J Surg 1994;18:70–75. 29. Silverstein MJ, Lagois MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med 1999;340:1455-1461. 30. Bartelink H, Horiot JC, Poortmans P, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001 Nov 8;345(19):1378-87. 31. Smith TE, Lee D, Turner BC, Carter D, Haffty BG. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000 Dec 1;48(5):1281-9. 32. Moon WK, Noh DY, Im GJ. Multifocal, multicentric, and contralateral breast cancers: bilateral whole-breast US in the preoperative evaluation of patients. Radiology 2002;224:569-576. 33. Orel SG, Schnall MD, Powell CM, et al. Staging of suspected breast cancer: effect of MR imaging and MR-guided biopsy. Radiology 1995;196:115-122. 34. Rosen PP, Fracchia AA, Urban JA, Schottenfeld D, Robbins GF. "Residual" mammary carcinoma following simulated partial mastectomy. Cancer 1975;35:739-747 35. Padera TP, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumour lymphatics. Science. 2002 Jun 7;296(5574):1883-6. 36. van de Vijver MJ, He YD, van 't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999-2009. 37. Roukos DH, Pavlidis N, Agnantis NJ. Gene-expression profile: the future in the outcome prediction and treatment of breast cancer. Gastric Breast Cancer 2003; 2(1): 5-8. 38. Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med 2002 Nov;347:1566-75. 39. Agnantis NJ, Roukos DH, Paraskevaidis E, Elisaf M. Cyclin E: a new biologic prognostic marker for breast cancer? Gastric Breast Cancer 2003; 2(1): 9-11. |
  |
|
