EDITORIAL
Circulating Cancer Cells: Limitations of Surgery and Need for Systemic Effective Treatment In Gastric Cancer
Angelos M. Kappas, MD and Dimitrios H. Roukos MD
From the Department of Surgery, Ioannina University School of Medicine, 45110 Ioannina, Greece,
droukos@cc.uoi.gr
Despite declining incidence, the mortality of patients diagnosed with gastric cancer remains high. Surgery with curative potential is the treatment of choice. Although postsurgical risk (morbidity, mortality) has been remarkably reduced in the recent years (mortality < 5%), there has been moderate progress in the improvement of overall long term survival rates. Estimates suggest that among all patients presenting with gastric cancer only approximately 10% to at best 30% in the West are still alive 5 years later.[1-3] Undoubtedly a chance for long-term survival is provided only by complete tumor removal (R0 resection) by surgery, whereas, unfortunately for all other patients, i.e., with noncurative resection, unresected, or with distant metastatic disease, survival is extremely poor[1]. But even after curative surgery, recurrence is often in the West[1-4] and Japan[5-6] and nearly always fatal.[1-6] Because adjuvant chemotherapy or radiotherapy has been of little or no efficacy,[1] the best way to improve outcome is to increase the rate of curative resection by detecting tumors early and to perform appropriate surgery to reduce the rate of recurrence. Indeed, using this strategy in Japan have increased the rates of R0 resection to about 80% now and overall survival to between 50% and 60%[5]. The rate of curative surgery in the West is still small ranging between 30% and 70%.[1-4]
Recurrence is the main cause of treatment failure following curative gastrectomy for cancer. Frequency of recurrence, time to first recurrent event and survival are strongly depended on the stage of disease at the time of surgery and extent of surgical resection. The debate over the last two decades continues over the optimal extent of surgery between limited (D1) and extended (D2) lymph node dissection. Two European randomized trials[2,3] failed to resolve this debate since the issue is complicated by many variables that had not taken into account. According to a recently described concept,[7] it is estimated that approximately 30% of all patients who are suitable for a potential curative surgery suffer from positive nodes around celiac axis (level II: stations 7 to 12).[1-3,5] This fact clearly indicates both the necessity of D2 resection for an R0 resection and the insufficiency of D1 resection that results in residual disease and fatal outcome in most, and perhaps all, patients with level II disease.[7]
This argument and the favorable studies on the outcomes of D2 resection from Japan[5] and several specialized institutions in the West[1] have convinced many investigators for the effectiveness of D2 resection for selected node-positive cancers. However, the adverse effect of D2 resection on postsurgical morbidity and mortality in the randomized trials suggest caution. The D2 technique should not be combined with pancreaticosplenectomy and performed by surgeons who are not familial with the technique.
Classification according to the International Union against Cancer (UICC) accurately predicts overall survival but is unable to provide information in terms of overall recurrence, time-specific recurrence-appearance and site-specific first recurrence. These valuable data is the basis for the development of causative recurrence-oriented and site-specific target therapies. The complexity of recurrence patterns involving locoregional, nodal, hematogenous, peritoneal and combinations of these recurrence-types[1,4,6] and a lack of prospective controlled studies suggest the need for well-documented recurrent data gathered prospectively for identification of site-specific risk factors.
Despite these limitations, recent reports from the West[4] and Japan[6] consistently indicate several critical key points in the mode of recurrence following curative resection. First, pathological serosa state is the determinant and independent factor for prediction of site-specific recurrence because patients with serosa-positive cancer are more likely to develop peritoneal recurrence than those with serosa-negative cancers. The presence of nodal metastasis markedly increases the risk of recurrence for both types of serosa status. Second, recurrence-risk following standardized technique with gastrectomy and extended (D2) lymph node dissection, is strongly depended on the tumor stage; low risk for both serosa and node- negative cancer (about 10%), but high risk in up to 85%[4] for both serosa and node-positive cancer. Postoperative adjuvant chemotherapy or radiotherapy has been proven of little or no efficacy in reducing recurrence and improving survival.[1] In a preliminary report of the Intergroup 116 US trial, it was suggested that some survival benefit may occur with chemoradiation after curative surgery. These findings should be approached with caution because many patients enrolled in the trial had an unacceptable limited (D0) surgery and survival rates in the treatment group are much lower or similar to that of surgery alone from Japan5 and Europe.[1-3] The effectiveness of neo-adjuvant treatment with pre-operative and/or intra-operative intra-peritoneal chemotherapy based on a numerous of theoretical advantages is now being testing in ongoing randomized trials.[1] In general however, there is doubt over an overoptimism about cancer and expectations for large survival benefits from new agents-based combination regimens in the next few years is unrealistic.[8]
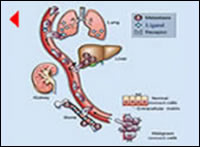
Why and how can it be explained that most (70%) patients develop a secondary tumor (recurrence) early (within two years)[1,4,6] following complete tumor resection? Circulating tumor cells have been identified in peripheral blood even at earlier tumor stages by current molecular techniques (reverse transcriptase-polymerase chain reaction (RT-PCR).[9] This fact strongly supports the theory that clinically occult tumor cells released from the original tumor before9 and during surgical maneuvers[10] enter the blood circulation, migrate and form metastatic tumors in distant target organs. Previous experimental studies have shown that changes in tumor cell kinetics occur within 24h of the original tumor removal and a week later measurable tumor size could be observed.[11] Therefore, these cells proliferate and invade surrounding tissues in the critical early postsurgical period and they subsequently become clinically detectable as secondary tumors (recurrence) in the target organs (peritoneum, liver) in the early follow-up period. The recent controversy over prognostic significance of circulating tumor cells[9] underlines the complexity of metastatic process. For example most recent molecular research for breast cancer reveal that mediator molecules (ligand-receptor pair) regulate the metastatic process in the target organs.[12,13] Because the significance of these molecules is probably not unique to breast cancer, one could speculate that similar or other molecules also regulate the metastatic process for gastric cancer.
These molecular-based findings explain and strongly indicate the limitations of surgery alone in reducing recurrence of advanced gastric cancer. Intense efforts and much work is needed toward development of effective combination regimens based on new agents. Molecular research will lead to the identification of small molecules for a target-selective therapy that added to chemotherapy will reduce the risk of site-specific recurrence. At present however, appropriate extent of surgery determined by nodal status with less or more extensive lymph node dissection for node-negative or node-positive cancers respectively should be performed with a standardized technique. This may reduce recurrence-risk and improve outcome.
References
1. Roukos DH, Fatouros M, Xeropotamos N, Kappas AM. Treatment of gastric cancer: early-stage, advanced-stage cancer, adjuvant treatment. Gastric Breast Cancer 2002; 1(1): 12-22.
2. Bonnenkamp JJ, Hermans J, Sasako M, van de Velde CJH, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340: 908-14.
3. Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resection for gastric cancer: long-term results of the MRC randomised surgical trial. Surgical co-operation group. Br J Cancer 1999; 79: 1522-30.
4. Lorenz M, Roukos DH, Karakostas K, Hottenrott C, Encke A. Accurate prediction of site-specific risk of recurrence after curative surgery for gastric cancer. Gastric Breast Cancer 2002; 1(2): 23-32.
5. Fujii M, Sasaki J, Nakajima T. State of the art in the treatment of gastric cancer: from the 71st Japanese gastric cancer congress. Gastric Cancer 1999; 2:151-7
6. Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer 2000; 89: 255-61.
7. Roukos DH. Optimising lymph lode dissection for gastric cancer. Gastric Breast Cancer 2002; 1(2): 40-43.
8. Editorial, The Lancet. Overoptimism about cancer. Lancet 2000; 355: 157.
9. Zippelius A, Pantel K. RT-PCR-based detection of occult disseminated tumor cells in peripheral blood and bone marrow of patients with solid tumors. An overview. Ann N Y Acad Sci 2000; 996: 110-123.
10. Miyazono F, Natsugoe S, Takao S, et al. Surgical maneuvers enhance molecular detection of circulating tumor cells during gastric cancer surgery. Ann Surg 2001; 233:189-94.
11. Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. CancerRes1979;39:3861-3865.
12. Liotta L. Cancer: An attractive force in metastasis. Nature2001;410:24-5.
13. Eisenhauer EA. From the molecule to the clinic - inhibiting HER2 to treat breast cancer. NEJM 2001; 344: 841-2.