Stomach carcinoma is still a major health problem. Despite declining incidence worldwide, mortality from diagnosed gastric cancer remains high. In the U.S., 12,800 deaths and 21,700 new gastric cancer cases are expected in 2001. Surgery resulting in complete tumor removal, namely R0 resection, according to the International Union Against Cancer (UICC1), remains the only effective treatment. Unfortunately, even following curative surgery, recurrence is frequent and always fatal. Despite great efforts over the past decades, postoperative adjuvant treatment including various chemotherapy regimens has failed to reduce recurrence risk and to improve survival rates.[2] Total gastrectomy combined with splenectomy and extended lymph node dissection has been proposed by Japanese surgeons for proximal gastric cancer treatment.[3] This approach has been performed with wider indication as standard procedure for all resected patients in several western institutions[4] for better local control and survival.
Subsequent observational studies failed to demonstrate any advantages of splenectomy[5-7] and moreover, others report an adverse effect of splenectomy on postoperative shortterm and/or long-term outcome,[8-13] but the data are still inconclusive. This controversy and the reliance of surgeons worldwide on the necessity of splenectomy to achieve an R0 resection are likely explanations for the high rate of splenectomy still observed.[12-14]
The uncertainty behind this dilemma of resection- overpreservation of the spleen, prompted us to conduct this prospective study.Omega replica watches For the design of this study we considered several critical key points for a validate assessment of this challenging topic, based on an our previous observation that the incidence of metastasis to the splenic hilum nodes was low and there was a trend toward better survival although insignificant for spleenpreservation patients.[15] Because splenectomy may promote a surgical stress-induced immunosuppression, we thought we would have an increased likelihood of detecting a causal association between presence of the spleen and suppression of recurrence-development from micrometastatic disease in the critical early postoperative period by choosing to evaluate early recurrence in particular, but also overall recurrence and survival.
Patients and eligibility criteria Patients were eligible if they had a histologically confirmed gastric carcinoma and had undergone a surgical resection. From February 1986 to December 1992, all patients who underwent gastrectomy alone (spleen-preservation group) or combined with resection of the spleen (splenectomy group) in the Department of Abdominal and General Surgery at Johann-Wolfgang-Goethe University Hospital in Frankfurt, Germany, were included in this prospective study.
Surgery
Resection of the spleen during gastrectomy was optional. Total gastrectomy with extended lymph node dissection was the treatment of choice. Extended (D2) lymph node dissection was performed with a systematic and standardized pancreas-preserving technique according to the slightly modified guidelines of the Japanese Research Society for Gastric Carcinoma (JRSGC16): D2 node dissection entailed, the removal of perigastric compartment I nodes (stations 1 to 6: D1 dissection) and the extraperigastric compartment II nodes including those around the celiac axis (stations 7 to 9), along the splenic artery (station 11), to the splenic hilum (10) and in ligamentum hepatoduodenal (station 12).
Pathology and Quality Control for Appropriate Patient Stratification
All diagnostic, surgical and histopathological data prospectively documented in a standardized protocol were used for an accurate stratification of patients according to curability of resection (R-stratification: R0 or R1/R2), extent of lymph node dissection (D1 or D2), tumor site (proximal vs. distal half of the stomach), type of gastrectomy (total vs. subtotal), histological type of Lauren classification (intestinal vs. diffuse)[17] and its clinical value,18 as well as nodal status (negative vs. positive), serosal status (serosa-negative vs. positive) and tumor-node-metastasis (TNM) staging system.[1]
Surgery was defined as curative if at laparotomy there was no macroscopic evidence for distant metastasis or suspected enlarged lymph nodes beyond the compartment II, the resection resulted in complete tumor removal with histologically proven tumor-free status in all resection margins in the final histological examination. A resection which did not fulfill all of these criteria was defined as non-curative (R1,R2 resection).
Because D1 dissection is associated with residual D2 positive nodes and thus is inaccurate for R-stratification, estimates of recurrence and survival were focused on D2 R0 subgroup. The pathology report of lymph node examination was used to control the surgical report for a complete D2 resection.
None of the patients treated curatively underwent adjuvant chemotherapy or radiotherapy and thus surgery alone is responsible for reported results.
After surgery we screened the patients using clinical examination, laboratory tests, chest radiography and abdominal ultrasound every 3 months, and endoscopic examination and computed tomography every 6 months. After the third year follow-up was done at 1-year intervals. The follow-up was completed at the end 1999. We recorded first recurrence and deaths from any cause.
Statistical Analysis
The primary endpoints were recurrence-free survival (recurrence), gastric cancer-specific survival (death from recurrence) and overall survival (death from any cause); the secondary endpoints, short-term postoperative outcome (morbidity, mortality) and frequency of metastasis to the splenic hilum lymph nodes. Recurrence or death from the disease, whichever occurred first, was separated into early recurrence (tumor appearance during the first 2 years) and overall recurrence (tumor at any time). All time periods up to the event (recurrence, death, last follow-up visit) were calculated from the date of surgery. The time-to-event endpoints were estimated using the Kaplan and Meier method and differences between the groups were compared with the log-rank test.
The relative risks of recurrence and death were calculated with the Cox proportional hazard model (univariate analysis).
Data were analyzed according to a prospectively defined plan. Primary analysis included all resected patients (intent-to-treat principle). All further estimates of recurrence and survival were focused on R0 D2 subgroup. Because it was expected that splenectomy would be performed more often in advanced tumor stages, leading in a significant imbalance, we planned a predefined adjustment and subgroup analysis according to these well-known prognostic baseline variables (nodal/serosal status).
We prospectively planned a multivariate analysis including all stratified factors at baseline (tumor site, stage, type and spleen-preservation) in Cox's model which would be proved significant by univariate analysis (log-rank test) to estimate the independent effect of these variables on outcomes. For statistical analyses, we used SPSS software for Windows (version 10.0).
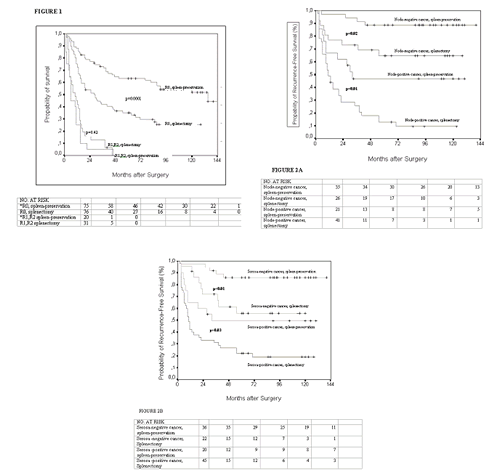
Overall survival for all resected patients 202 of the 210 resected patients were followed-up on; 95 had gastrectomy with preservation of the spleen and 107 had gastrectomy combined with splenectomy. Overall, the presence over absence of the spleen was associated with better overall survival (p=0.0003 by the log-rank test) and was associated with a decreased risk of death from any cause [HR, 0.66 (95% CI 0.46-0.95; p=0.02)] in a multivariate Cox regression analysis independently of the standard prognostic factors, including pathological, node stage (pN), tumor depth (pT), curability of resection (R), and extent of lymph node dissection (D). A subgroup analysis, however, reveals that the presence of the spleen improved survival only among patients (n=151) who had an R0 resection in innadjustment (p=0.0001) and adjustment for tumor stage (p=0.008) analysis whereas there was no such effect on patients (n=51) with noncurative surgery. Despite resection survival for these patients with residual disease after resection was poor; mean survival time 10 months (95% CI, 5 to 14) for spleen preservation patients and 13 months (95% CI, 9 to 18) for splenectomized patients (p=0.42).
Curative Gastrectomy With Extended (D2) Lymph Node Dissection
Of the 146 patients who fulfilled our criteria for a D2 resection, 126 met the criteria for stratification into the R0 group; 59 had gastrectomy alone (spleen-preservation group) and 67 underwent gastrectomy combined with resection of the spleen (splenectomy group). Table 1 lists the baseline characteristics. There was a significant imbalance in stratification factors related to prognosis; a higher distribution of less advanced nodal stage (pN, p=0.02) and tumor depth (pT, p=0.001) in the spleenpreservation group.
View this figure:
|
Table 1: *The tumor-node-metastasis classification of the Union International Contre le Cancer (UICC) and The American Joint Committee on Cancer, 4th Edition was used.1
+ The nodal stage of the Japanese Research Society for Gastric Cancer, 1st English ed. Was used1,2 the abbreviation pT, pN denote pathologically confirmed tumor-nodes.Because of rounding, not all percent ages total 100.
|
Short-term postoperative outcome
There was no difference between preservation versus resection of the spleen in postoperative septic complications (anastomotic leakage, intra-abdominal infection, wound infection; 6 [10%] and 8 [12%]) or overall significant complications (11 [18.6%] and 13 [19.4%]) or frequency of re-operations (5 [8.5%] and 5 [7.5%]. There was also no difference in hospital mortality between the spleen-preservation group (2 [3.4%]) and splenectomy group (0%).
Recurrences
Of 126 patients, 2 died postoperatively in hospital and one was lost to follow-up. We estimated recurrence risk among 123 R0, D2 patients. The median length of follow-up was 55 months for all 123 patients and 112 months for survivors. Gastric cancer recurred in 15 of 56 (27%) spleen-preservation patients compared with 45 of 67 (67%) splenectomized patients (Table 1). This difference in the rate of recurrence was significant in the early follow-up period (early recurrence; p<0.001 in inadjustment and p=0.003 in adjustment analysis for nodal status) or at any time after surgery (overall recurrence) [Table 2]. This favorable effect of the spleen was also consistently observed in the prospectively defined subgroup analyses with a reduction in the relative risks of overall recurrence ranging from 52% to 74% among the prognostic important subgroups at baseline (node, serosa negative/positive cancers) (Table 2).
View this figure:
|
Table 2: + At risk for evaluation of recurrence where 123 of 126 patients who received a curative D2 resection and left the hospital; 2 patients died postoperatively in-hospital and one was lost to follow-up.
‡ Splenectomy group served as the reference group. CI denotes confidence interval.
* The Coxproportional hazard model was applied. Relative risk less than 1.00 represents an increased risk of recurrence or death.
§ Hazard ratio was used to calculate P values.
This analysis included 125 patients; one was lost to follow-up. All deaths irrespective of cause, including postoperative in-hospital deaths, were included.
|
Kaplan-Meier analyses of recurrence-free survival yielded similar results in inadjustment analysis (p<0.0001), adjustment analyses for nodal status (p=0.0008) and serosa status (p=0.001), and subgroups analyses.
Survival
In all, 72 patients died, 24 in the spleen-preservation group and 48 in the splenectomy group. The causes of death were recurrent gastric cancer in 60 patients (83%), other diseases with no evidence of recurrence in 10 (14%) and postoperative complications in 2 (3%). All 60 patients who recurred died shortly after recurrence manifested (median survival time only 5 months). Thus, the reduction in the risk of death from gastric cancer was similar to that of recurrence (Table 3 ).
View this figure:
|
Table 3:* Hazard ratios less than 1.00 represent a decreased risk, whereas greater than 1.00 represent an increased risk CI denotes confidence interval.
|
Compared with splenectomy, preservation of the spleen significantly lowered the risk of death from any cause and improved overall survival in inadjustment (p==0.001), and adjustment analysis (p=0.009 for nodal status and p=0.01 for serosa-status) (Table 2). The reduction in the relative risks of death from any cause among the 4 predefined subgroups [node, serosa (negative/positive) cancers) were statistically marginal significant or insignificant (Table 2).
Prospectively-defined subgroup analyses for several baseline variables listed in Table 1, confirmed the prognostic significance of pathological nodal status (p<0.0001) and serosal status (p<0.0001), but did not show any significant difference with respect to the site of the primary tumor (upper vs. middle vs. distal third of the stomach; p=0.67) and the histological-type according to Lauren classification (intestinal vs. diffuse-type, p=0.14).
In a Cox's model which included the prognostic significant factors, the proportional-hazards analysis reveals that preservation over resection of the spleen was associated with significantly reduced risks of recurrence or death from gastric cancer by 58 % (95% CI, 0.23 to 0.76); p=0.005) and death from any cause by 47% (95% CI, 0.32 to 0.89; p=0.01), independently of pathological nodal status and serosal status (Table 3).
Risks resulting from preservation of the spleen
Presence of metastases in the splenic hilum lymph nodes was evident in only 4 of 67 R0 D2 splenectomized patients (6%), whereas no metastasis into the spleen or tumor invasion through serosa to the spleen was found. Of the 117 lymph nodes retrieved from the hilum of the spleen (mean nodal yield per specimen 1.7 nodes (range 0- 4); 6 were positive.
Analysis of variables thought to be associated with increased risk of metastasis to the splenic hilum nodes revealed: a) all four patients with positive splenic hilum nodes had a tumor located in the proximal half of the stomach (4/30, 13%) which had penetrated the serosa (pT3-cancer, 4/ 45, 9%) and b) 3 of these 4 patients had a tumor in the greater curvature (3/30, 10%) and also 3 had positive several of the other compartment II nodes (3/21, 14%). All 4 had an early recurrence (3, 11, 12 and 13 months after surgery) and died within 2 years.
In this study, preservation of the spleen as compared with splenectomy during curative gastrectomy for ancer, given similar postsurgical risks (morbidity/mortality), substantially reduced the risks of recurrence and death from any cause. A survival benefit was evident throughout the 10-year follow-up period, regardless of whether the preservation and resection of the spleen groups were stratified according to tumor stage.
Treatment of gastric cancer by total resection of the stomach en-block with the spleen for increasing local curability[3,4] has failed to fulfil expectations of better survival.[5-7] Moreover, in several clinical reports, an adverse effect of splenectomy on postsurgical risks (complications, mortality)[5-8,10,12,13] as well as on long-term survival was demonstrated by univariate analysis.[7-11] However, since in none of these reports splenectomy could be identified as an independent predictor of poor survival,7- 9 effect of the spleen on long-term survival remains controversial.[2]
So, why this consistent effect of the spleen in our study, and why are our findings in stark contrast with the continual reliance on splenectomy demonstrated by surgeons worldwide and currently reflected in a high rate of splenectomy, ranging from 26% to 50%?[11-14]There are several explanations.
Firstly, because spleen effect on recurrence is limited to R0 patients, and because recurrence risk evaluation prerequisites a curative surgery, a validate assessment should ensure an accurate R0-stratification. This was feasible in our prospectively defined D2 R0 group, based on standardized criteria. D1 resection is associated with a high proportion of residual positive D2 nodes (30%)[19] and thus is inappropriate for such evaluation.
Secondly, this is the first study focused on evaluation of early recurrence. In estimating the risks of early recurrence, we increased the probability to detect a significant treatment effect of the spleen because over 75% of recurrences occur within 2 years after R0 surgery.[20] We assessed that by an early recurrence rate of 75% (45/60), the presence of the spleen decreased the risks of early recurrences in adjustment for nodal status analysis by 67% (95% CI, 0.16 to 0.69; p=0.003)
Thirdly, a consistent effect of spleen-preservation was seen among the 4 prospectively predefined subgroups (node negative/positive, serosa negative/positive cancers) with a well-known prognostic significance. This evaluation is necessary because of a significant imbalance of these factors (nodal/serosal status) at baseline.[21] These findings are consistent with that of the largest (n=3477) available study from the U.S.[11] Wanebo et al. based on obtained significant survival differences in favor of spleen preservation in tumor stages II and III, propose preservation of the spleen in these stages.[11] Despite that in the study by Wanebo et al. as also in our study, a better but not significant survival difference in stage I was found, we think that the spleen should also be preserved for patients with early tumor stage I, because there is no case of presence of splenic hilar nodal metastasis. A finding that holds great clinical importance and emphasize the significance of spleen-preservation in our study is that recurrence-free survival and overall survival was similar among patients with node-positive cancers and spleen-preservation and those with node-negative cancers and splenectomy. The treatment effect was also consistent with multivariate analysis in which spleenpreservation was an independent predictor of outcome.
Fourthly, our own study reflects an overestimation of the residual tumor risk with spleen-preservation. Splenectomy, performed in 53% of the cases, was actually needed for an R0 resection in only 6%, since metastases in the splenic hilum nodes was found in 4 of 67 D2 R0 patients, whereas no metastasis into the spleen or tumor invasion through serosa to the spleen was detected. Our lymphatic-spread findings are consistent with Japanese experience,[22] indicating that in practice, only patients with proximal serosa-positive cancers are at risk of having positive splenic hilar nodes. Indeed, in a most recent study from Europe this incidence was low (9.8%)[14] even among proximal advanced stages (UICC IIIb, IV).[23] But even with that limitations accurate prediction among these patients (18% in this study) is challenging.
At present, information about primary tumor site and stage (patients with proximal, serosa positive cancers at greater curvature with other D2 positive nodes are likely at higher risk), may be useful for prediction and splenectomy decision, until new diagnostic methods or data from large trials establishing a prediction model can provide evidence of nodal status at the splenic hilum. The consistent reduction observed of early recurrences and mortality among spleen-preservation patients should be attributable to the suppression of growth of occult disseminated tumor cells. The presence of these cells at the time of curative surgery has been demonstrated by current diagnostic techniques (immunocytochemically, reverse transcriptase-polymerase chain reaction (RTPCR)].[24] Under a surgical stress-induced immunosuppression, previously shown and more recently confirmed,[25] changes in minimal residual tumor cell kinetics after curative surgery with rapid tumor growth have been experimentally demonstrated on critical early postoperative time.[26] Though the role of the spleen in tumor immunology is still unclear, some molecular research findings (T-cells and NK cell activity, immunosuppressive acidic protein (IAP), specific antitumor reactivity by stimulation of spleen cells with MAGE peptide)[27-30] support our hypothesis that the presence of the spleen enhances an antitumor immune response of the host resulting in suppression of recurrencedevelopment from minimal residual tumor. To expect massive mortality reduction in the near future by testing new drugs is likewise unrealistic.[31] For gastric cancer various postsurgical adjuvant treatment modalities tested over the last three decades have proved of little or no efficacy. By contrast, our results have direct implication for most patients worldwide receiving gastrectomy for cancer. Preservation of the spleen during curative surgery in this study substantially reduced recurrence risk and improved survival rates. Since we were able to eliminate bias from significant imbalance of tumor stage at baseline by prospectively predefined adjustment and subgroup analyses, we believe that the spleen should be preserved in most patients receiving curative gastrectomy.
1. Bearhs OH, Henson DE, Hutter RVP, Kennedy BJ, editors. Manual for staging. American Joint Committee on Cancer. 4th edition. Philadelphia: J. B. Lippincott, 1992.
2. Roukos DH, Fatouros M, Xeropotamos N, Kappas AM. Treatment of gastric cancer: early-stage, advanced-stage cancer, adjuvant treatment. Gastric Breast Cancer 2002; 1(1): 12-22.
3. Marujama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 1987; 11:418-25.
4. Jahne J, Meyer HJ, Maschek H, Geerlings H, Bruns E, Pichlmayr R. Lymphadenectomy in gastric carcinoma. Arch Surg 1992; 127: 290-4.
5. Adachi Y, Kamakura T, Mori M, Maechara Y, Sugimachi K. Role of lymph node dissection and splenectomy in node positive gastric carcinoma. Surgery 1994; 116: 837-841
6. Otsuji E, Yamaguchi T, Sawai K, Okamoto K, Takahashi T. Total gastrectomy with simultaneous pancreaticosplenectomy or splenectomy in patients with advanced gastric carcinoma. Br J Cancer 1999; 79: 1789-93.
7. Kwon SJ. Prognostic impact of splenectomy on gastric cancer: results of the Korean gastric cancer study group. World J Surg 1997; 21: 837-844.
8. Brady MS, Rogatho A, Dent LL, Shiou MH. Effect of splenectomy on morbidity and survival following curative gastrectomy for gastric carcinoma. Arch Surg 1991; 26: 359-364.
9. Stipa S, DiGiorgio A, Ferri M, Botti C. Results of curative gastrectomy for carcinoma. J Am Coll Surg 1994; 17: 567- 572
10. Griffith JP, Sue-Ling HM,, Dixon MF, McMahon MJ, Axon ATR, Johnston D. Preservation of spleen improves survival after radical surgery for gastric cancer. Gut 1995; 36: 684- 690
11. Wanebo HJ, Kennedy BJ, Winchester DP, Stewart A, Fremgen M. Role of splenectomy in gastric cancer surgery: adverse effect of elective splenectomy on long-term survival. J Am Coll Surg 1997; 185: 177-184.
12. Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resection for gastric cancer: long-term results of the MRC randomised surgical trial. Surgical cooperation group. Br J Cancer 1999; 79: 1522-30.
13. Bonnenkamp JJ, Hermans J, Sasako M, van de Velde CJH, et al. Extended lymph node dissection for gastric cancer. N Engl J Med 1999; 340: 908-14.
14. Schmid A, Thybusch A, Kremer B, Henne-Bruns D. Differential effects of radical D2-lymphadenectomy and splenectomy in surgically treated gastric cancer patients. Hepatogastroenterology 2000; 47: 579-85.
15. Roukos D, Hottenrott C, Lorenz M, Encke A. Gastric carcinoma - Gastrectomy with or without splenectomy depending tumour stage and localisation. Akt Chir 1989; 24: 96-101 (German).
16. Nishi M, Omori Y, Miwa K, editors. Japanese classification of gastric carcinoma. Japanese Research Society for Gastric Cancer (JRSGC). 1st English edition. Tokyo: Kanehara & Co., 1995.
17. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 1965;64:31-49.
18. Roukos DH, Agnantis NJ, Fatouros M, Kappas AM. Gastric Cancer: Introduction, pathology, epidemiology. Gastric Breast Cancer 2002; 1(1): 1-3.
19. Roukos DH. Extended (D2) lymph node dissection for gastric cancer: do patients benefit? Ann Surg Oncol. 2000 May;7(4):253-5. Editorial.
20. Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer 2000; 89: 255-61.
21. Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000; 365: 1064-69.
22. Okajima K, Isozaki H. Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg 1995; 19: 537-40.
23. Monig SP, Collet PH, Baldus SE et al. Splenectomy in proximal gastric cancer: Frequency of lymph node metastasis to the splenic hilum. J Surg Oncol 2001; 76: 89- 92.
24. Zippelius A, Pantel K. RT-PCR-based detection of occult disseminated tumor cells in peripheral blood and bone marrow of patients with solid tumors. An overview. Ann N Y Acad Sci 2000; 996: 110-123.
25. Ogawa K, Hirai M, katsube T, et al. Suppression of cellular immunity by surgical stress. Surgery 2000; 127: 329-36.
26. Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res 1979; 39: 3861-3865.
27. Wakasugi T, Takeda T, Monden , et al. Augmentation of splenic antitumor immunity by local immunotherapy in gastric cancer patients. Biotherapy 1997; 10: 99-106.
28. Fujie T, Tanaka F, Tahara K, et al. Generation of specific antitumor reactivity by the stimulation of spleen cells from gastric cancer patients with MAGE-3 synthetic peptide. Cancer Immunol Immunother 1999; 48:189-94.
29. Okuno K, Tanaka A, Shigeoka H, ewt al. Suppression of T-cell function in gastric cancer patients after total gastrectomy with splenectomy: implications of splenic autotransplantation. Gastric Cancer 1999; 2:20-25.
30. Saji S, Sakamoto J, Teramukai S, et al. Impact of splenectomy and immunotherapy on survival following gastrectomy for carcinoma: covariate interaction with immunosuppressive acidic protein, a serum marker for the host immune system. Surg Today 1999; 29: 504-10.
31. Editorial, The Lancet. Overoptimism about cancer.Lancet 2000; 355: 15.