|
Effectiveness of Adjuvant Chemoradiotherapy in Patients with Gastric Cancer Treated with Less Extensive Surgery
Dimitrios H. Roukos, MD
From the Department of Surgery, Ioannina University School of Medicine, 45110 Ioannina, Greece,
droukos@cc.uoi.gr
|
Worldwide, gastric cancer despite declining incidence remains one of the most common malignancies and a leading cause of cancer death. Although in the USA only 22,700 new cases are expected in 2001 real case-mortality remains high indicating little progress in the treatment of diagnosed gastric cancer. However, hopes for an improved patient outcome provides a most recent report from USA.[1] This large, carefully monitored, randomized trial is clinically important because up until now there was no credible evidence for the effectiveness of adjuvant treatment after gastric resection for cancer.[2-4] |
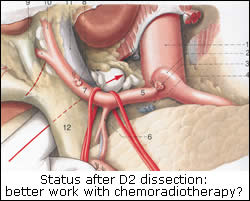 |
In this multi-institutional trial, 556 patients after resection for adenocarcinoma of the stomach or gastroesophageal junction were randomly assigned to surgery plus postoperative chemoradiotherapy or surgery alone. The adjuvant treatment consisted of fluorouracil plus leucovorin followed by 4500 cGy of radiation. Chemoradiotherapy improved significantly the median overall survival by 9 months. The hazard ratio for death was 1.35 (95 percent confidence interval, 1.09 to 1.66; P=0.005). Three patients (1%) died from toxic effects of the chemoradiotherapy; grade 3 and grade 4 toxic effects occurred in 41% and 32% of the patients in the chemoradiotherapy group respectively. Macdonald and his colleagues concluded that postoperative chemoradiotherapy should be considered for all patients at high risk for recurrence of adenocarcinoma of the stomach or gastroesophageal junction who have undergone curative resection.
Given that most (90%) patients studied had undergone limited (D0 or D1) lymph node dissection and only 10% had undergone extended (D2) lymph node dissection, two key questions emerge: (1) should all patients, including those who had undergone D2 dissection, receive adjuvant chemoradiotherapy? (2) Is a multidisciplinary approach consisted of limited (D0 or D1) lymph node dissection plus this adjuvant treatment more safe and effective than D2 resection alone? We need a working hypothesis to approach these questions. According to an our recently described concept[5] limited node dissection, as compared with extended dissection, is associated with substantially increased risk of residual positive nodes. This risk can accurately be calculated by studying established histopathological data among patients who had undergone a curative D2 dissection. Analysis of these data from Japan[6,7] and western world[8,9] consistently indicates that about 30%[4,10] among patients who had a D2 dissection proved curative, had cancer disease in the extraperigastric level II nodes (around the celiac axis and in hepatoduodenal ligament; N2 disease according to the Japanese classification). These extraperigastric nodes are left behind after D1 dissection. Consequently, since 90% of the patients in the study by Macdonald et al. had a D0 or D1 node dissection, at least one third had residual disease after surgery. If we look at treatment plan of the study we assess that radiation was delivered to the tumor bed and to these regional nodes which were left behind by limited surgery in the study. Therefore it is likely that these certain patients with N2 disease are those who benefited from adjuvant treatment. Thus reliable is the question whether control of the disease in the regional lymph nodes can be better achieved by chemoradiotherapy or by surgical resection.
Comparison of treatment-related morbidity and mortality between D2 dissection alone and combined treatment consisted of limited surgery plus adjuvant chemoradiotherapy is useful for treatment decision. Evidence for the safety of D2 dissection provides a preliminary report of a most recent Japanese well designed and conducted multi-institutional randomized trial.[11] The study demonstrates a low risk of mortality (1%) and morbidity (about 20%) associated with extended (D2 or D4) lymph node dissection. These data are consisted with that of many nonrandomized studies from specialized institutions[5-7,12] and confirm the safety of D2 dissection when it is performed with a systematic and standardized pancreas-preserving technique by experienced surgeon. This lack of D2-txhnique experienced surgeons in the randomized trials was the main reason[14] for the high mortality (>10%) of D2 dissection occurred in these two European randomized trials.[7,8] These D2 dissection -related complications rates are much lower than the major toxic effects occurred very frequently (54% hematologic, 33% gastrointestinal) in chemoradiotherapy group if we add the limited-surgery related complications which are not reported.
Although comparison of safety between these two treatments is feasible and valid, there are substantial limitations for effectiveness-related comparison. The 3-year survival rate of 50% in the chemoradiotherapy group is clearly worse or similar to the 5-year survival rates recently reported after D2 dissection from Japan (over 60%),[6] from western specialized institutions (around 50%)[5,11] and the Dutch randomized trial (47%).[7] However, it is hard to drawn conclusions from such a long-term survival comparison between East and West because of differences in patients characteristics and tumor biological behavior and particularly of differences in the distribution of early and advanced stages. Indeed, in the chemoradiotherapy study the proportion of patients with more advanced stages, which undoubtedly associated with poor survival, was higher than in all other studies.
There is no doubt that the goal in gastric cancer management is the complete removal of the primary tumor and of the affected regional lymph nodes (curative resection). If the disease is localized but it has spread to the extraperigastric lymph nodes this goal seems to be attainable only by extended lymph node dissection. This goal cannot be achieved by limited D0 or D1 dissection that results in a high rate, over 40%, of local and nodal recurrence, while after D2 dissection this failure rate can drastically be reduced in than 12%.[13] It is likely therefore, that postoperative chemoradiotherapy was effective in D0 or D1 patients reducing local and nodal recurrences and improving survival. However, the data of the USA trial are unable to suggest either a survival benefit of adjuvant chemoradiotherapy after D2 dissection or a replacement of D2 dissection by this combined treatment as a more safe and effective treatment.
These questions can be addressed by future randomized trial, but at present D2 dissection is the treatment of choice when it can be adequately be performed.
Juicy Couture Outlet www.cheapjuicyoutlets.com
References
1. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345: 725-30.
2. Hermans J, Bonenkamp JJ, Boon MC, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomised trials. J Clin Oncol 1993; 11: 1441-1447.
3. Hallissey MT, Dunn JA, Ward LC, et al. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet 1994; 343:1309-12.
4. Roukos DH, Fatouros M, Xeropotamos N, Kappas AM. Treatment of gastric cancer: early-stage, advanced-stage cancer, adjuvant treatment. Gastric Breast Cancer 2002; 1(1): 12-22.
5. Roukos DH, Lorenz M, Encke A. Evidence of survival benefit of extended (D2) lymphadenectomy in western patients with gastric cancer based on a new concept: a prospective long-term follow-up study. Surgery 1998 May;123(5):573-8.
6. Fujii M, Sasaki J, Nakajima T. State of the art in the treatment of gastric cancer: from the 71st Japanese gastric cancer congress. Gastric Cancer 1999; 2:151-7.
7. Bonnenkamp JJ, Hermans J, Sasako M, van de Velde CJH, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340: 908-14.
8. Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resection for gastric cancer: long-term results of the MRC randomised surgical trial. Surgical co-operation group. Br J Cancer 1999; 79: 1522-30.
9. Roukos DH. Optimising lymph lode dissection for gastric cancer. Gastric Breast Cancer 2002; 1(2): 40-43.
10. Sano T, Sasako M. Randomized controlled trial to evaluate para-aortic lymphadenectomy for gastric cancer (JCOG 9501): An update. 4th International Gastric Cancer, New York 2001, Abstracts, S45, p.663.
11. Siewert JR, Boettcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer. Ten-year results of the German Gastric Cancer Study. Ann Surg 1998; 228: 449-461
12. Brennan MF. Lymph-node dissection for gastric cancer. New Engl J Med 1999;340:956-8 (editorial).
13. Lorenz M, Roukos DH, Karakostas K, Hottenrott C, Encke A. Accurate prediction of site-specific risk of recurrence after curative surgery for gastric cancer. Gastric Breast Cancer 2002; 1(2): 23-32.
|


